Critical review on “pyrin” protein: its role in NLRP3 inflammasome activation
2015/01/14
Abstract
Pyrin, protein mutated in Familial Mediterranean Fever (FMF), can regulate inflammasome complexes. Different agonists of pyrin inflammasome are established which drive ASC-dependent caspase-1 activation. Because of a structural discrepancy between human and murine pyrin, the protein's functional role is debated. To understand what drives FMF, it is necessary to understand whether WT pyrin has an anti- or pro-inflammatory role. Different mouse models (truncated, entire Mefv locus-deficient) support an inhibitory role of pyrin whilst the recent exon 2 Mefv KO mice favours pyrin's role in acting as a pattern recognition receptor thus highlighting its key role in mammalian innate immunity to respond to various bacterial modifications of Rho GTP-ases. Pyrin also triggers an inflammosome assembly upon bacterial stimuli of B. cenocepacia, F. novicida. It inhibits NLRP3 inflammasome activation, but initiates a formation of its own caspase-1 activating inflammasome complex. KI mouse studies showed that NLRP3 has no role in FMF disease.
Table of Contents
Introduction
The burden of autoinflammatory diseases is increasing as well as its morbidity and mortality. These disorders are defined as unprovoked inflammation in the absence of autoantibodies and antigen-specific T cells [1]. Understanding mechanisms which drive the inflammation through macromolecular complexes such as inflammasomes has been an intense field of study to develop therapeutics. Inflammasomes detect tissue stress and pathogens and trigger signalling pathways to secrete pro-inflammatory cytokines [12].
There is a wide spectrum of autoinflammatory disorders varying from rare monogenic, such as Familial Mediterranean Fever (FMF), and usual multifactorial diseases like Crohn's disease [2]. FMF and Cryopyrin Associated Periodic Syndrome (CAPS) account for mutations in pyrin and cryopyrin proteins. Pyrin's mutation in FMF is well established but its function is still elusive. Moreover, FMF has gained an increasing attention lately due to its severe clinical features and rising prevalence not only in the Mediterranean basin but also in some European countries. The review aims to reveal how pyrin regulates NLRP3 inflammasome, to identify whether pyrin's role is involved in a positive or negative regulation of caspase‑1 activation as well as to illustrate how it leads to an autoinflammatory disease given controversial mechanistic hypotheses.
Pyrin protein
Pyrin, protein mutated in FMF, is encoded by a MEFV (MEditerranean FeVer) gene. This 781-amino acid protein is mainly expressed in neutrophils, monocytes, and dendritic cells, but not in lymphocytes [3]. All missense mutations causing FMF are clustered in B30.2 domain (Fig.1) that is involved in protein-protein interactions [4]. ASC, an adaptor protein
found in all inflammasomes, interacts with pyrin, a sensor molecule, via PYD‑PYD cognate interactions [5]. ASC contains two domains: PYD and a caspase activation and recruitment domain (CARD). Due to its CARD, ASC can activate caspase‑1, which further activates pro-inflammatory interleukin‑1 beta (IL‑1β) [6] (Fig.2). So, it is assumed that pyrin's interaction with ASC results in a pyrin's regulatory role in the inflammasome. Normally, pyrin is not part of the NLRP3 inflammasome (Fig.2). Because it is not recognized as a NOD‑like receptor although it has its PYD domain [7]. The way pyrin interacts with NLRP3 protein complex will be further discussed. Also, pyrin can form its own pyrin inflammasome where it serves as a sensor of pathogens or cellular stress which is also covered in the review.
Mutations in two copies of MEFV alleles are attributed to the 85% of FMF patients, whilst up to 25% of patients with clinically apparent features carry mutation in one copy with the majority being healthy[8]. The study of pyrin's physiological role is challenging. Because murine pyrin is dissimilar to human pyrin; the former lacks B30.2 domain which includes all FMF-associated mutations [9] (Fig.1). It makes difficult to extrapolate data from the mouse pyrin to explain the molecular mechanisms of FMF pathogenesis.
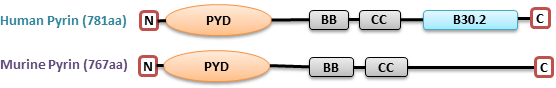
Figure 1. Comparison of human pyrin with mouse pyrin. .
Human pyrin: N-terminal PYD (pyrin) domain, two B-box (BB) zink finger, coiled-coil (CC), C-terminal B30.2 domain. Mouse pyrin: PYD, BB, CC domains.
MEFV gene
In 1997, MEFV gene was identified on a chromosome 16p using positional cloning. It coded for a 781-amino acid protein named pyrin in mature granulocytes[10]. Independently, French FMF Consortium discovered transcriptional units of 60kB in the FMF gene MEFV: among 4 genes found, they termed one to be marenostrin (Latin mare nostrum- Mediterranean sea), whose missense variations results in FMF disease [11].
NLRP3 inflammasome
NLRP3 (NLR‑based PYD domain containing 3) is a multiprotein cytosolic complex which senses pathogen- or danger-associated molecular patterns (PAMPs, DAMPs) and like other inflammasomes defend against pathogen infection by caspase‑1 activation. PAMPs vary from bacterial peptidoglycans to viral DNAs [12] whereas DAMPs are host-derived signals[13] such as extracellular ATP or potassium efflux. NLRP3 complex activation requires both PAMP and DAMP in human monocytes and mouse macrophages [9].The inflammasome proteins possess either PYD or CARD domains thereby resulting in homotypic interactions through PYD‑PYD and CARD‑CARD assembly (Fig.2). There are other non‑NLR proteins like AIM‑2 [14] and pyrin [7] which also stimulate AIM‑2 and pyrin inflammasome formation. Normally, inflammasome activation is manifested by caspase‑1 activation and IL‑1β secretion. Its overstimulation is responsible for the pathogenesis of different autoinflammatory disorders.
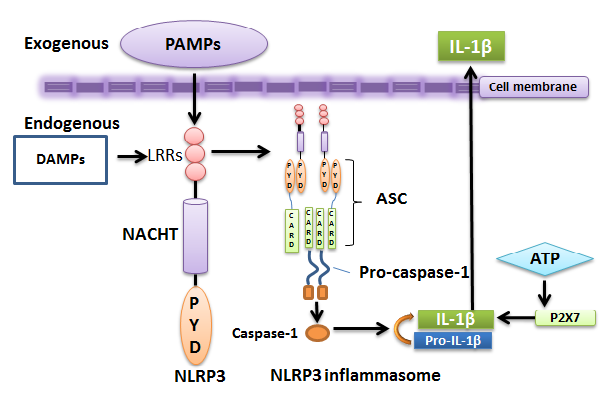
Figure 2. NLRP3 inflammasome..
The structure consists of CARD-containing caspase-1, CARD- and PYD-containing ASC protein, and an intracellular sensor nucleotide-binding oligomerization domain receptors, in short, NOD-like receptors (NLRs). NLRs act as sensors of PAMPs/DAMPs. An NLR, here NLRP3, represents C-terminal leucine-rich repeat (LRR), central nucleotide domain (NACHT), and N-terminal effector PYD domain [12]. NLRP3 inflammasome is formed when NLRP3 binds to an ASC proteinthrough its PYD domain. ASC subsequently recruits the procaspase-1 via CARD-CARD interactions.Inflammasome assemblyinitiates caspase-1 activationwhich regulates maturation of pro-inflammatory cytokine interleukin-1 beta (IL-1β) and its secretion. IL-1β secretion can be also stimulated by an additional ATP signal through the nucleotide receptor P2X7 [15]. Secreted IL-1β serves as a main performer for tissue destruction and pyroptosis [16].
Pyrin in human diseases
Familial Mediterranean fever (FMF)
FMF (OMIM 249100) is the most common hereditary autoinflammatory syndrome caused by missense mutations in the B30.2 domain of the pyrin protein [17]. FMF has a high prevalence (1/200 - 1/1000) in the south-east Mediterranean area. Pyrin regulates caspase‑1 activation. Loss-of-function MEFV mutations possibly cause autoinflammation through reduction of pyrin's inhibitory role which leads to IL‑1β production[18] whilst gain-of-function mutation arises in a dominant‑like pattern of inheritance of the disease which has an autoinflammatory phenotype, too [19]. FMF is manifested by recurrent episodes of fever attacks with local inflammation and joint pain in the absence of detectable pathogenic stimulus [20].
PAPA syndrome
Autoinflammatory syndrome of pyogenic arthritis, pyoderma gangrenosum, acne (PAPA) accounts for missense mutations in cytoskeleton-organizing protein PSTPIP1 [21]. PAPA syndrome is similar to FMF even though the former's clinical features are more severe causing tissue destruction. Mutant PSTPIP1 has a greater affinity for pyrin's BB and CC domains, thus leading to an indirect inflammasome activity [22].
FMF epidemiology
Age: FMF disease manifests itself during the early ages: 50% of incidence before the age of 10 and 90% before the 20 years old.
Ethnicity: 200 MEFV mutations have been dated in a population inhabiting the Mediterranean basin: Turks, Armenians, Jewish, Arabs, Italians and North Africans. Today, FMF spread is not only limited to this area, but also worldwide [28] thanks to milder MEFV mutations (V726A).
Environmental factors: Living in a country, susceptible for the disease, could be a risk factor: 78% of FMF patients in Turkey had severe symptoms as opposed to 34% living in Germany, a country with better living conditions [29].
Treatment: Colchicine is effective in reduction of FMF attacks and amyloidoisis [30]. Those who are non-responsive to the colchicine treatment, they are alternatively treated with IL-1 antagonist (anakinra) or the human IL‑1β monoclonal antibody (canakimumab) [31]. All these treatments reduce the frequency of attacks.
Other human diseases
Pyrin mutation (M694V) has been recently associated with ankylosing spondylitis, an autoimmune disease, in the Chinese Han population [23]. Severe autosomal-dominant periodic autoinflammatory disorder with AA amyloidosis was shown to result from the H478Y MEFV mutation which halts the functional role of pyrin [24]. Giaglis et al. [25] report that 7 (n=25) ulcerative colitis (UC) patients had MEFV mutations. Moreover, MEFV mutations also need to be studied in patients with Crohn's disease (CD). Because inflammatory bowel diseases are reported to be frequent in FMF families [26]. So far, extraintestinal lesions caused by CD have been associated with MEFV mutations [27]. It is implied that high frequency of MEFV mutations in UC patients with inflammatory arthritis gives an altered effect in the course of the disease and enables MEFV to localize in the joints. Further confirmation is needed with a larger patient series.
Hypotheses of mutated pyrin's effect in FMF
There exist contradictory hypotheses regarding mutated pyrin's role in FMF pathogenesis: pyrin inflammasome hypothesis which supports pyrin's inflammatory role, and sequestration hypothesis which supports pyrin's anti-inflammatory role (Fig.3). According to the former, FMF manifests as a subsequence of a gain-of-function mutation (M694V, M680I, V726A) in pyrin's B30.2 which enables it to be constitutively active in the absence of triggers thereby forming an inflammasome whilst the latter explains FMF emergence as a result of pyrin's loss-of-function mutation that leads to pyrin's failure to sequester ASC so that caspase‑1 gets activated thus inducing inflammation.
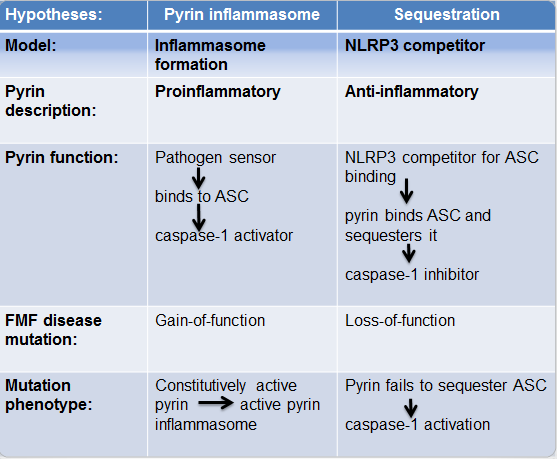
Figure 3. Different models suggesting pyrin function and its respective mutations in causing FMF. .
Pyrin inflammasome hypothesis proposes that pyrin forms an inflammasome by detecting PAMPs via its B30.2 domain, thereby activating caspase-1 [32]. FMF results due to a chronically active pyrin inflammasome. Sequestration hypothesis suggests that pyrin competes with NLRP3 for ASC-binding thus inhibiting downstream IL-1β processing upon ASC sequestration [5,33]. FMF results due to pyrin's inability to sequester ASC which leads to an enhanced IL-1β secretion.
Anti-inflammatory role of pyrin
In overexpression studies pyrin was reported to negatively regulate caspase‑1 by competitively binding to ASC[34] and leaving less availability of ASC for NLRP3[35]. In particular, pyrin's B30.2 domain is able to bind directly to caspase‑1 and hinder its activation. Furthermore, FMF mutations clustered in this domain had a reduced binding to caspase‑1 which resulted in IL‑1β secretion. Also, pyrin downregulation in THP‑1 cells increased IL‑1β secretion, an indicator of the inflammasome activation[33]. Studies performed in mouse models favour pyrin's inhibitory role [5,36].
Pyrin inhibits NLRP3-mediated inflammation
NLRP3 or cryopyrin, encoded by the NLRP3 gene, is a protein mutated in inflammatory diseases as familial cold urticaria and Muckle-Wells syndrome [37]. Normally, NLRP3 binds ASC via PYD homotypic interactions. Pyrin also binds ASC. Pyrin‑ASC interaction was found to suppress NLRP3‑mediated NF‑kB activation via disruption of NLRP3‑ASC collaboration [34]. Such an elucidation of pyrin's inhibition of NF‑kB firstly suggested its inhibitory role in the formation of the NLRP3 inflammasome so that FMF-associated pyrin mutations stimulate enhanced ASC signaling causing the inflammation.
Another study found out that pyrin‑ASC dialogue activates NF‑kB signaling when pyrin was coexpressed in HEK293T cells [38]. But, it was later refuted by demonstrating that neither pyrin nor its FMF mutations does not induce NF‑kB activity in cells with endogenous ASC expression[7]. The conflicting results account for using different cell types: ASC overexpression in transfected cells contrary to 293‑ASC cells with stable ASC numbers.
To continue, pyrin is reported to disrupt NLRP3 inflammasome activation (Fig.4) due to its interference with the NLRP3 complex which limits IL‑1β processing. Pyrin's B30.2 domain interacts with NLRP3, caspase‑1 and the inflammasome substrate, IL‑1β.
![Pyrin negatively regulates NLRP3 inflammasome (adapted from Papin et al. [35]).](Pictures/m2-14-15-biosci-reviews-duisembekova-a-1c-Fig4.png)
Figure 4. Pyrin negatively regulates NLRP3 inflammasome (adapted from Papin et al. [35]). .
Pyrin interacts with NLRP3 sensor via its B30.2 and PYD domains which bind respectively to NACHT and PYD domains of NLRP3 in the inflammasome complex. This inhibits pro-IL-1β processing. Furthermore, pyrin binds to pro-IL-1β, negatively affecting pro-IL-1β processing.
Pyrin inhibits inflammasome via ASC sequestration
Pyrin's role in vivo was attempted to be studied in mouse models. A mouse model was generated by deleting C‑terminal of murine pyrin so that it consists of only PYD domain [5]. Because mouse with a truncated pyrin suffered from high body temperatures (>39C), it was believed to mimic the FMF features. Moreover, the mice had elevated IL‑1β levels in macrophages in response to bacterial lypopolysaccharide (LPS), which led to a hypothesis that WT pyrin negatively regulates inflammasome. So, an anti-inflammatory role of pyrin implies that WT murine pyrin binds to ASC causing its sequestration so that ASC‑caspase‑1 interaction is not allowed. Consequently, caspase‑1 activation is suppressed. These results can be argued since pyrin had its C‑terminal deleted that acts as a regulatory domain. However, deletion of a regulatory domain in Ipaf and NLRP3 which belong to NLR family resulted in a generation of active proteins [39]. So, its deletion in mice does not indicate a mutant pyrin to arrive to pyrin's inhibitory role. Moreover, macrophages in mice with pyrin truncation might have disrupted apoptosis compared to WT upon LPS treatment. It is also possible that increased sensitivity to LPS is due to an accumulation of macrophages in pyrin-truncated mice. Furthermore, murine pyrin lacks the B30.2 domain so these observations cannot be attributed to explain FMF pathogenesis.
Pyrin inhibits caspase‑1 activation
Pyrin was found to suppress caspase‑1 activation by binding to its catalytically active sites (p10 and p20) via B30.2 domain [33]. This was supported by computational docking analyses, and FMF-associated mutations in B30.2 (M680I, M694V, V726A) demonstrated less binding with caspase‑1. FMF mutations are thought to affect pyrin‑caspase‑1 interaction based on overexpression studies. In contrast, Papin et al. [35] did not observe consistent dampened interaction of pyrin with M694V mutation with caspase‑1. Interestingly, B30.2‑caspase‑1 binding was found to be ASC-independent[33]. Yu and his colleagues[7] proved an absolute necessity of ASC for pyrin to initiate caspase‑1 on transfection system stably expressing ASC. Surprisingly, pyrin missing the B30.2 domain but retaining its PYD domain had a significant increase in IL‑1β secretion [33]. In the absence of ASC, it is expected that no PYD‑PYD interaction between pyrin and ASC takes place. Consequently, an inhibition of IL‑1β processing should have been observed.
Loss of murine pyrin increases IL‑1β levels
The entire Mefv locus-deleted mice had an increased NLRP3 inflammasome-mediated IL‑1β release in response to LPS and elicitors (Alum, ATP) in resident peritoneal macrophages (RPM) [36]. These results are conflicting with findings by Chae et al. [17] who observed that IL‑1β release by bone marrow-derived macropages (BMDMs) was not different when stimulated with LPS or ATP in a KI mouse model of FMF, generated by fusing human B30.2 domain to a mouse pyrin. These deviations account for two factors: utilization of different cells such as RPMs in the former which has more pyrin; and BMDMs in the latter, which has much less pyrin; and different mouse models, KO and KI. It is noteworthy that KO mouse has no overt FMF phenotype whilst KI model generated almost similar to or severe phenotype of FMF. KI mice results suggest that activation of caspase‑1 was ASC-dependent but NLRP3-independent, thus ruling out the necessity of NLRP3 in the FMF induction. Loss of murine pyrin did not only impact the activation of NLRP3 inflammasome complex, but also involved other NLRC4 and NLRP1b inflammasomes' assembly upon challenge with their respective elicitors (flagellin and Bacillus anthracis) [36].
As it can be seen, it is not possible to extrapolate results obtained from mouse for FMF that makes the gene function comparison complicated. It raises a necessity to develop new methods to address the challenge.
The targeted deletion of exon 2 of mouse Mefv enabled the generation of TALEN-mediated Mefv-/- KO mouse [40] which manifested that pyrin acts as a pattern recognition receptor (PRR), thus supporting pyrin's inflammatory role provided below.
Proinflammatory role of pyrin
Observations that human pyrin is primarily stimulated as an immediate-early gene by cytokines [18] and all the FMF mutations are found in B30.2 domain argue for an inflammatory role of pyrin. Pyrin positively regulates caspase‑1 when overexpressed in 293T cells stably expressing ASC and caspase‑1 [7]. Furthermore, pyrin increases IL‑1β production in response to LPS [41], Francisella novicida,Burkhrolderia cenocepacia infections [42,43], mutant PSTPIP1 which causes PAPA syndrome [22], and ribotoxic stress [44]. Moreover, pyrin was regarded as a PRR in BMDMs to detect bacterial induced Rho subfamily inactivation induced by virulence factors of C.difficile, C.botulinum in pyrin KO mice to trigger ASC-dependent caspase‑1 activation, extended IL‑1β secretion in BMDMs. Together, these data suggest a stimulatory role of pyrin in inducing inflammation and these are discussed below.
ASC-dependent pyrin inflammasome assembly
Pyrin positively regulates caspase‑1 by binding to ASC and provoking the ASC oligomerization. ASC is crucial in recruitment and autoprocessing of procaspase‑1. Consequently, pyrin-ASC complex is a prerequisite for ASC-dependent caspase‑1 recruitment. So, pyrin acts as a proinflammatory protein [7]. In contrast, Chae et al. [5] support pyrin's anti-inflammatory role suggesting that pyrin causes ASC sequestration, thereby achieving inflammasome inhibition. It is noteworthy that the latter observations were obtained using a mouse model whose pyrin had a C‑terminal truncation [5] whilst a pro-inflammatory role of pyrin (Fig.5) was established using human pyrin and 293T cells expressing ASC and procaspase‑1 at physiological levels in such a way that artificial results are eliminated due to the protein levels' variations
Both pyrin and NLRP3 form independently pyrin(Fig.5) and NLRP3 inflammasome complexes with ASC and procaspase‑1 where caspase‑1 activation is based on ASC oligomerization. More interestingly, pyrin ‑NLRP3 dialogue can result in a slight increase of ASC-dependent caspase‑1 activation. So, ASC plays an absolute role both in pyrin and NLRP3‑mediated caspase‑1 induction. To sum up, it is implied that FMF-associated mutations modify pyrin's activity and give rise to increased oligomerization of ASC.
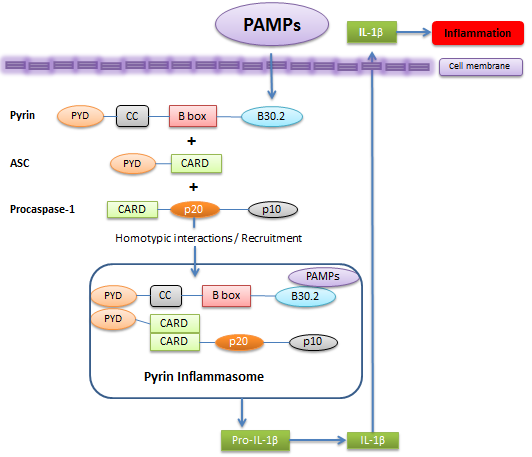
Figure 5. Pyrin positively regulates inflammasome. .
In this suggested model, pyrin's B30.2 domain acts as a regulatory motif which senses PAMPs. This results in pyrin's binding to and oligomerization of ASC, and recruiting caspase-1. These form a pyrin inflammasome complex which further processes IL-1β causing inflammation.
Pyrin's role in ASC pyroptosome activation
Pyrin's interaction with PSTPIP1, protein mutated in PAPA syndrome, regulates IL‑1β signaling via ASC[45]. Initially, pyrin rests in a homotrimeric autoinhibited state due to its PYD domain bound to B‑box. PYD remains hidden, and this limits pyrin's interaction with ASC. PSTPIP1 can unmask PYD by binding to pyrin's BB domain, thus rendering its active conformation. Pyrin's interaction with ASC is achieved followed by ASC oligomerization and downstream IL‑1β generation. Mutant PSTPIP1 was observed to have a higher affinity for BB domain of pyrin later forming an ASC pyroptosome, a supramolecular complex comprising of dimerized oligomers of ASC and caspase‑1 recruitment [32]. So, pyrin is absolutely required to sense PAPA-associated PSTPIP1 mutants to stimulate ASC-dependent caspase‑1 activation with according IL‑1β upregulation by acting as a direct activator of ASC pyroptosome. This sheds a light on a molecular mechanism of PAPA syndrome. Importantly, pyrin's CC‑domain is found to be critical for PSTPIP1-regulated activities. Because it is responsible for pyrin's homotrimerization. The latter is crucial for pyrin's capacity to trigger ASC oligomerization to activate caspase‑1. Even more significantly, pyrin is regarded as a pro-inflammatory molecule after viewing how colchicine inhibited caspase‑1 activation mediated by pyrin upon challenge with PSTPIP1 mutants.
Pyrin initiates inflammasome assembly upon sensing bacteria
Human pyrin recognizes intracellular bacteria Burkholderia cenocepacia in human monocytes[43]. Pyrin's regulation of inflammasome was shown: depletion of pyrin levels led to a significant reduction in caspase‑1 and mature IL‑1β secretion in response to B.cenocepacia whereas pyrin's overexpression resulted in more IL‑1β release. This is supported by the Wewer's lab [41] where pyrin knockdown led to a diminished IL‑1β processing in fresh human monocytes, too. To continue, ASC-dependent inflammasome activation was identified where ASC overexpression provoked significantly more IL‑1β secretion. Moreover, ASC‑pyrin interactions were established in infected cells. So, Gavrilin et al. [43] were the first to demonstrate that pyrin is absolutely required to detect B.cenocepacia due to its bacterial type VI secretion system (T6SS). This resulted in pyrin's forming an inflammasome. Besides, B.cenocepacia induces the pyrin inflammasome activation by modifying RHOA subfamily: it deamidates asparagine‑41 in the switch‑I region via T6SS that is detected by pyrin [40].
Pyrin's level is important to sense bacteria and induce inflammation: pyrin deficit in human monocytes and monocyte-derived macrophages (MDMs) accounted for a decreased IL‑1β release upon F.novicida infection [42]. Normally, there is a high expression level of pyrin in human monocytes but it falls with the cells' differentiation into MDMs. Pyrin levels can be easily restored in MDMs by treating them with macrophage colony-stimulating factor (M‑CSF) [42]
Constant maintenance of pyrin expression in MDMs with Francisella stimulus allows for inflammasome assembly by activating caspase‑1. But, it is also possible that M‑CSF induces inflammasome through pyrin-independent regulators since it is capable of activating several signaling pathways [46]. Also, a knockdown of pyrin in THP‑1 cells did not affect pro‑IL‑1β and the mature IL‑1β mRNA levels whilst there was a significant reduction in IL‑1β release. This might be due to either a disruption or a lack of an additional signal like ATP that is necessary for IL‑1β secretion (Fig.2). It also concerns MDMs which are reported to have an inflammasome impairment due to their incapacity of IL‑1β release upon infection although they express IL‑1β at a similar rate as human monocytes. All in all, Francisella acts as an agonist for pyrin via binding to a B30.2 domain followed with a B‑box interaction and unmasking of PYD domain so that it homotypically interacts with ASC. Hence, determination of pyrin's role as a unique sensor of the pathogen which drives inflammation is in agreement with a pro-inflammatory role of pyrin.
Furthermore, recently pyrin was reported to sense ribotoxic stress [44], a response initiated as a result of damage to 28S ribosomal RNA that further activates p38 MAPK signaling [47] and Rho modification by toxins [40]. Pyrin-dependent caspase‑1 activation in response to MAPK signaling and bacterial inactivation of Rho‑GTPases reinforce pyrin's role as a mediator of inflammasome assembly.
Different results supporting either a proinflammatory or anti-inflammatory role of pyrin account for using different cell types and transfection studies. Pyrin's inhibition of caspase‑1 is achieved by myeloid and monocyte cell lines [33,35] which endogenously express all the molecules to observe the IL‑1β processing whilst pyrin's activation of caspase‑1 is observed in HEK‑293T cells which are more advantageous in terms of facilitated transfection in tissue culture. Although HEK‑293T cells are stably transfected with ASC, they cannot recapitulate all of the endogenous protein interactions in leukocytes compared to the human myeloid and monocytic cell lines.
Conclusion
As of the interaction between pyrin and NLRP3 inflammasome, pyrin was found to interfere with NLRP3 negatively regulating IL‑1β processing. Instead, pyrin forms its own inflammasome to sense PAMPs acting as a positive caspase‑1 regulator based on the results in human cell lines. In my opinion, mutations of pyrin in FMF patients are supposed to have a gain-of- function character. During evolution, these mutations are hypothesized to confer sensitivity to an extensive range of PAMPs than the WT pyrin which recognizes a single one [7]. Currently, the pathogen is not yet identified. Since FMF is inherited in an autosomal recessive pattern, the heterozygotes are conferred a selective advantage against the pathogen and that is why they are healthy whereas the homozygous suffer from increased inflammatory response. Together, these data support a pro-inflammatory role of pyrin.
Acknowledgments
I thank Dr Bénédicte Py for her critical reading of the review, Professor Bertrand Mollereau for his useful comments, and Dr Antoine Corbin for provision of the technical support during the review writing.
References and recommended reading
Papers of particular interest have been highlighted as:
[1] McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, Mansfield E, Gadina M, Karenko L, Pettersson T, et al.: Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 1999, 97:133–144. [PubMed]
[2]Touitou I: Inheritance of autoinflammatory diseases: shifting paradigms and nomenclature. J. Med. Genet. 2013, 50:349–359. [Full Paper][PubMed]
[3]Diaz A, Hu C, Kastner DL, Schaner P, Reginato AM, Richards N, Gumucio DL: Lipopolysaccharide-induced expression of multiple alternatively spliced MEFV transcripts in human synovial fibroblasts: a prominent splice isoform lacks the C-terminal domain that is highly mutated in familial Mediterranean fever. Arthritis Rheum. 2004, 50:3679–3689. [Full Paper][PubMed]
[4]Woo J-S, Imm J-H, Min C-K, Kim K-J, Cha S-S, Oh B-H: Structural and functional insights into the B30.2/SPRY domain. EMBO J. 2006, 25:1353–1363. [Full Paper][PubMed][PubMed Central]
[5]Chae JJ, Komarow HD, Cheng J, Wood G, Raben N, Liu PP, Kastner DL: Targeted disruption of pyrin, the FMF protein, causes heightened sensitivity to endotoxin and a defect in macrophage apoptosis. Mol. Cell 2003, 11:591–604. [PubMed]
[6]Martinon F, Tschopp J: Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell 2004, 117:561–574. [Full Paper][PubMed]
[7]Yu J-W, Wu J, Zhang Z, Datta P, Ibrahimi I, Taniguchi S, Sagara J,
Fernandes-Alnemri T, Alnemri ES: Cryopyrin and pyrin activate
caspase-1, but not NF-kappaB, via ASC oligomerization. Cell
Death Differ. 2006, 13:236–249.
[Full
Paper][PubMed] ●● Authors were the first to use a different cell system, HEK293
cell-based reconstitution system, to establish a proinflammatory role of pyrin.
Pyrin was found to form its own inflammasome complex upon sensing pathogens.
Caspase-1 activation was found to be ASC-dependent both in pyrin and NLRP3
inflammasome complexes.
[8]Lachmann HJ, Şengül B, Yavuzşen TU, Booth DR, Booth SE, Bybee A, Gallimore JR, Soytürk M, Akar S, Tunca M, et al.: Clinical and subclinical inflammation in patients with familial Mediterranean fever and in heterozygous carriers of MEFV mutations. Rheumatology 2006, 45:746–750. [Full Paper][PubMed]
[9]Ozkurede VU, Franchi L: Immunology in clinic
review series; focus on autoinflammatory diseases: role of inflammasomes in
autoinflammatory syndromes. Clin. Exp. Immunol.
2012, 167:382–390. [Full
Paper][PubMed][PubMed Central] ● Authors give a concise and a very comprehensive overview on two
autoinflammatory diseases: CAPS and FMF. The importance of inflammasome and its
constituent proteins in the pathogenesis of these disorders is widely described
as well as providing different hypotheses as of mutant cryopyrin and pyrin's
role in the disease causation.
[10]The International FMF Consortium: Ancient Missense Mutations in a New Member of the RoRet Gene Family Are Likely to Cause Familial Mediterranean Fever. Cell 1997, 90:797–807. [Full Paper]
[11]French FMF Consortium: A candidate gene for familial Mediterranean fever. Nat. Genet. 1997, 17:25–31. [Full Paper][PubMed]
[12]Martinon F, Mayor A, Tschopp J: The inflammasomes: guardians of the body. Annu. Rev. Immunol. 2009, 27:229–265. [Full Paper][PubMed]
[13]Schroder K, Tschopp J: The
inflammasomes. Cell 2010, 140:821–832. [Full
Paper][PubMed] ●● A very detailed comprehensive review explaining mechanisms of function
of NLRP3, NLRP1, IPAF and AIM-2 inflammasomes, providing their potential
agonists as well as covering regulatory molecular mechanisms that drive the
activation or suppression of each inflammasome.
[14]Roberts TL, Idris A, Dunn JA, Kelly GM, Burnton CM, Hodgson S, Hardy LL, Garceau V, Sweet MJ, Ross IL, et al.: HIN-200 proteins regulate caspase activation in response to foreign cytoplasmic DNA. Science 2009, 323:1057–1060. [Full Paper][PubMed]
[15]Netea MG, Nold-Petry CA, Nold MF, Joosten LAB, Opitz B, van der Meer
JHM, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, et al.: Differential requirement for the activation of the inflammasome for processing and
release of IL-1beta in monocytes and macrophages.
Blood 2009, 113:2324–2335.
[Full Paper][PubMed][PubMed Central] ● The paper describes an importance of an additional ATP signal required
for secretion of IL-1β, generated by the NLRP3 inflammasome, onto the cell
membrane to further mediate inflammation.
[16]Walsh JG, Muruve DA, Power C: Inflammasomes in the CNS. Nat. Rev. Neurosci. 2014, 15:84–97. [Full Paper][PubMed]
[17]Chae JJ, Cho Y-H, Lee G-S, Cheng J, Liu PP, Feigenbaum L, Katz SI, Kastner DL: Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1β activation and severe autoinflammation in mice. Immunity 2011, 34:755–768. [Full Paper][PubMed][PubMed Central]
[18]Centola M, Wood G, Frucht DM, Galon J, Aringer M, Farrell C, Kingma DW, Horwitz ME, Mansfield E, Holland SM, et al.: The gene for familial Mediterranean fever, MEFV, is expressed in early leukocyte development and is regulated in response to inflammatory mediators. Blood 2000, 95:3223–3231. [PubMed]
[19]Chae JJ, Wood G, Richard K, Jaffe H, Colburn NT, Masters SL, Gumucio DL, Shoham NG, Kastner DL: The familial Mediterranean fever protein, pyrin, is cleaved by caspase-1 and activates NF-kappaB through its N-terminal fragment. Blood 2008, 112:1794–1803. [Full Paper][PubMed][PubMed Central]
[20]Kastner DL, Aksentijevich I, Goldbach-Mansky R: Autoinflammatory disease reloaded: a clinical perspective. Cell 2010, 140:784–790. [Full Paper][PubMed][PubMed Central]
[21]Wise CA, Gillum JD, Seidman CE, Lindor NM, Veile R, Bashiardes S, Lovett M: Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum. Mol. Genet. 2002, 11:961–969. [PubMed]
[22]Shoham NG, Centola M, Mansfield E, Hull KM, Wood G, Wise CA, Kastner DL: Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc. Natl. Acad. Sci. U. S. A. 2003, 100:13501–13506. [Full Paper][PubMed][PubMed Central]
[23]He C, Li J, Xu W: Mutations in the B30.2 domain of pyrin and the risk of ankylosing spondylitis in the Chinese Han population: A case-control study. Immunol. Lett. 2014, doi:10.1016/j.imlet.2014.07.002. [Full Paper][PubMed]
[24]Aldea A, Campistol JM, Arostegui JI, Rius J, Maso M, Vives J, Yagüe J: A severe autosomal-dominant periodic inflammatory disorder with renal AA amyloidosis and colchicine resistance associated to the MEFV H478Y variant in a Spanish kindred: an unusual familial Mediterranean fever phenotype or another MEFV-associated periodic inflammatory disorder?. Am. J. Med. Genet. A. 2004, 124A:67–73. [Full Paper][PubMed]
[25]Giaglis S, Mimidis K, Papadopoulos V, Thomopoulos K, Sidiropoulos P, Rafail S, Nikolopoulou V, Fragouli E, Kartalis G, Tzioufas A, et al.: Increased frequency of mutations in the gene responsible for familial Mediterranean fever (MEFV) in a cohort of patients with ulcerative colitis: evidence for a potential disease-modifying effect?. Dig. Dis. Sci. 2006, 51:687–692. [Full Paper][PubMed]
[26]Cattan D, Notarnicola C, Molinari N, Touitou I: Inflammatory bowel disease in non-Ashkenazi Jews with familial Mediterranean fever. Lancet 2000, 355:378–379. [Full Paper][PubMed]
[27]Fidder H, Chowers Y, Ackerman Z, Pollak RD, Crusius JBA, Livneh A, Bar-Meir S, Avidan B, Shinhar Y: The familial Mediterranean fever (MEVF) gene as a modifier of Crohn's disease. Am. J. Gastroenterol. 2005, 100:338–343. [Full Paper][PubMed]
[28]Ben-Chetrit E, Touitou I: The impact of MEFV gene identification on FMF: an appraisal after 15 years. Clin. Exp. Rheumatol. 2012, 30:S3–6. [PubMed]
[29]Ozen S, Aktay N, Lainka E, Duzova A, Bakkaloglu A, Kallinich T: Disease severity in children and adolescents with familial Mediterranean fever: a comparative study to explore environmental effects on a monogenic disease. Ann. Rheum. Dis. 2009, 68:246–248. [Full Paper][PubMed]
[30]Taskiran EZ, Cetinkaya A, Balci-Peynircioglu B, Akkaya YZ, Yilmaz E: The effect of colchicine on pyrin and pyrin interacting proteins. J. Cell. Biochem. 2012, 113:3536–3546. [Full Paper][PubMed]
[31]Ozturk MA, Kanbay M, Kasapoglu B, Onat AM, Guz G, Furst DE, Ben-Chetrit E: Therapeutic approach to familial Mediterranean fever: a review update. Clin. Exp. Rheumatol. 2011, 29:S77–86. [PubMed]
[32]Fernandes-Alnemri T, Wu J, Yu J-W, Datta P, Miller B, Jankowski W, Rosenberg S, Zhang J, Alnemri ES: The pyroptosome: a supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007, 14:1590–1604. [Full Paper][PubMed][PubMed Central]
[33]Chae JJ, Wood G, Masters SL, Richard K, Park G, Smith BJ, Kastner
DL: The B30.2 domain of pyrin, the familial Mediterranean fever
protein, interacts directly with caspase-1 to modulate IL-1? production.
Proc. Natl. Acad. Sci. U. S. A. 2006, 103:9982–9987. [Full
Paper][PubMed][PubMed Central] ● Authors support an anti-inflammatory role of pyrin which inhibits IL-1β
processing by binding to catalytic active sites of caspase-1 via the B30.2
domain, thereby rendering caspase-1 inactive to process IL-1β.
[34]Dowds TA, Masumoto J, Chen FF, Ogura Y, Inohara N, Núñez G: Regulation of cryopyrin/Pypaf1 signaling by pyrin, the familial Mediterranean fever gene product. Biochem. Biophys. Res. Commun. 2003, 302:575–580. [PubMed]
[35]Papin S, Cuenin S, Agostini L, Martinon F, Werner S, Beer H-D,
Grütter C, Grütter M, Tschopp J: The SPRY domain of Pyrin, mutated
in familial Mediterranean fever patients, interacts with inflammasome components and
inhibits proIL-1beta processing. Cell Death Differ.
2007, 14:1457–1466. [Full
Paper][PubMed] ●● Authors show how pyrin disrupts NLRP3 inflammasome activation through
pyrin's B30.2 binding to NACHT domain of NLRP3 sensor in the caspase-1
activating complex. The suppression of IL-1β processing is further achieved by
pyrin-pro-IL-1β interaction, thereby favouring an inhibitory role of pyrin in
inducing inflammation.
[36]Hesker PR, Nguyen M, Kovarova M, Ting JP-Y, Koller BH: Genetic Loss of Murine Pyrin, the Familial Mediterranean Fever Protein, Increases Interleukin-1β Levels. PLoS ONE 2012, 7:e51105. [Full Paper]
[37]Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD: Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 2001, 29:301–305. [Full Paper][PubMed]
[38]Stehlik C, Fiorentino L, Dorfleutner A, Bruey J-M, Ariza EM, Sagara J, Reed JC: The PAAD/PYRIN-family protein ASC is a dual regulator of a conserved step in nuclear factor kappaB activation pathways. J. Exp. Med. 2002, 196:1605–1615. [PubMed][PubMed Central]
[39]Martinon F, Burns K, Tschopp J: The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol. Cell 2002, 10:417–426. [PubMed]
[40]Xu H, Yang J, Gao W, Li L, Li P, Zhang L, Gong Y-N, Peng X, Xi JJ,
Chen S, et al.: Innate immune sensing of bacterial modifications
of Rho GTPases by the Pyrin inflammasome. Nature
2014, 513:237–241. [Full
Paper][PubMed] ●● Pyrin's physiological role is shown to serve as a pattern recognition
receptor(PRR) in primary bone marrow-derived macrophages(BMDMs) to sense
bacterial induced Rho subfamily inactivation and to form pyrin inflammasome.
Pyrin recognizes modifications of Rho-GTPases driven by virulence factors of
C.difficile, H.somni, C.botulinum that leads to
Rho-glucosylation, adenylylation, ribosylation, respectively. These observations
were obtained by a generation of MEFV KO mice by TALEN system that is the best
pyrin KO mouse model as of now.
[41]Seshadri S, Duncan MD, Hart JM, Gavrilin MA, Wewers MD: Pyrin levels in human monocytes and monocyte-derived macrophages regulate IL-1beta processing and release. J. Immunol. Baltim. Md 1950 2007, 179:1274–1281. [PubMed]
[42]Gavrilin MA, Mitra S, Seshadri S, Nateri J, Berhe F, Hall MW, Wewers
MD: Pyrin Critical to Macrophage IL-1? Response to Francisella
Challenge. J. Immunol. Baltim. Md 1950 2009,
182:7982–7989. [Full
Paper][PubMed][PubMed Central] ● Pyrin was shown to detectt F.novicida bacteria in
monocytes inducing caspase-1 activation. It was discovered that macrophage
colony-stimulating factor(M-CSF) can restore pyrin levels in monocyte-derived
macrophages to defend against the bacterial infection by inducing formation of
inflammasome.
[43]Gavrilin MA, Abdelaziz DHA, Mostafa M, Abdulrahman BA, Grandhi J,
Akhter A, Khweek AA, Aubert DF, Valvano MA, Wewers MD, et al.: Activation of the Pyrin Inflammasome by Intracellular Burkholderia
cenocepacia. J. Immunol. Baltim. Md 1950 2012,
188:3469–3477. [Full
Paper][PubMed][PubMed Central] ● The authors were the first to find out another potential agonist of pyrin
B.cenocepacia. Pyrin detects Type VI secretion system of the bacteria in
mononuclear cells driving ASC recruitment and caspase-1 activation.
[44]Yu J-W, Farias A, Hwang I, Fernandes-Alnemri T, Alnemri ES: Ribotoxic stress through p38 mitogen-activated protein kinase activates in vitro the human pyrin inflammasome. J. Biol. Chem. 2013, 288:11378–11383. [Full Paper][PubMed][PubMed Central]
[45]Yu J-W, Fernandes-Alnemri T, Datta P, Wu J, Juliana C, Solorzano L,
McCormick M, Zhang Z, Alnemri ES: Pyrin activates the ASC
pyroptosome in response to engagement by autoinflammatory PSTPIP1
mutants. Mol. Cell 2007, 28:214–227. [Full
Paper][PubMed][PubMed Central] ● The paper provides evidence regarding pyrin's role as acting a sensor of
mutant PSTPIP1, protein mutated in PAPA syndrome, to form ASC pyroptosome which
is a platform for caspase-1 activation.
[46]Hamilton JA: Colony-stimulating factors in inflammation and autoimmunity. Nat. Rev. Immunol. 2008, 8:533–544. [Full Paper][PubMed]
[47]Kataoka T: Translation inhibitors and their unique biological properties. Eur. J. Pharmacol. 2012, 676:1–5. [Full Paper][PubMed]


