The scaffolding protein RACK1: multiple roles in human cancer
2013/01/21
Abstract
RACK1 is a ribosomal protein with a structure that allows a wide range of interaction with other proteins and that has diverse functions in model organisms. RACK1 was shown to take part in many signaling pathways, some of which being involved in tumorigenesis. Moreover, its ribosomal function makes of it a central regulator of cellular homeostasis. It is then supposed to be a hub between signaling pathways and ribosome. Here we summarize the reason why RACK1 may be involved in cancer progression by its action on proliferation, apoptosis, angiogenesis, migration, translation and miRNA efficiency.
Table of Contents
Introduction
Cancer is a lethal disease characterized by the abnormal proliferation of a single cellular clone that becomes immortal. It is described as a multistep and progressive process that enables cancerous cells to gain the ability to survive and to become invasive and malignant. These cells must acquire mutations that prevent them from undergoing apoptosis. Indeed, they do not respond to proapoptotic stimuli anymore. These cells can thus proliferate and induce angiogenesis. This process occurs as the tumor has not enough oxygen and nutrients to survive, and results in the sprouting of new vessels from existing ones. Moreover, cancer cells may invade other tissues and form metastasis. This process requires loss of matrix adhesion and cell-cell contacts, allowing cells to leave their original tissue. Once the new location is reached, the reverse process is occurring. In a recent review, Hanahan and Weinberg described ten hallmarks of tumor cells to rationalize cancers biology [1]••. Among these hallmarks, they included resistance to cell death, induction of angiogenesis, sustaining proliferative signal, invasion and metastasis potential; pre-existing functions that are turned away in the carcinogenic process.
Receptor for Activated C Kinase 1 (RACK1) plays a role in many cellular functions: it is involved in development, immune response, brain activity, addiction as well as in circadian rhythm [2,3]. It is also targeted by intracellular parasites as viruses and bacteria for cell subversion, showing its importance in cell regulation [4]•. RACK1 is involved in many signaling pathways as well as in translation and is a key factor of cellular homeostasis. Because of its multiple roles in cell activity, RACK1 may participate to the subversion of physiological functions, and lead to the acquisition of some hallmarks of cancer.
Interestingly, RACK1 expression has been shown to be upregulated in some cancers like pulmonary adenocarcinomas, hepatocellular carcinoma and metastatic melanoma [3]••. Moreover, it was supposed to be involved in ovarian and prostate cancers, as well as in Human Papilloma Virus 16 and Helicobacter pylori mediated neoplasia. Because of its procarcinogenic functions, it was proposed as a therapeutic target in colon cancer [5–8], or as a prognostic indicator in breast cancer [9,10]. All these involvements of RACK1 in human cancers tend to consider RACK1 as a potential interesting factor for the understanding and the treatment of cancer.
RACK1 structure allows a wide range of functions
A ribosomal docking protein conserved among species
RACK1 is a 36 kDa protein identified in 1994 as an anchoring protein for activated Ser/Thr kinase Protein Kinase C (PKC), mainly the PKCβII isoform, and is encoded by the human gene gnb2l1 [2]••. RACK1 has also been described as a compound of translating ribosomes [4]•. Crystallographic studies show that RACK1 is composed of tryptophane and aspartic acid (WD)-repeats (Figure 1) that adopt a seven bladed propeller structure, allowing protein-protein interaction. In its ribosome-bound form, one face of RACK1 exposes its WD-repeats as a docking platform [11]. The fact that RACK1 interacts with many other proteins than PKC is thus not surprising. RACK1 is involved in activated PKC localization and may play a role in the spatial distribution of signals. Increasing evidence suggests that biological response specificity may be controlled by signaling localization and that RACK1 may take part in this process. Moreover, this protein is evolutionarily conserved throughout eukaryotes (Figure 1), suggesting it carries important physiological functions [12].
In addition to its possible role in coordinating signaling pathways, it may play a role in mRNA translation. RACK1 is a ribosomal protein that is present at the small ribosomal subunit, near to the mRNA exit channel [11]. This privileged location may allow RACK1 to participate to ribosomes translational selectivity. However, even if RACK1 is now known to be a ribosomal protein [4]••, most of the knowledge on this protein is focused on its cytoplasmic form. Indeed, this ribosomal protein can also be found in its free form in the cytoplasm.
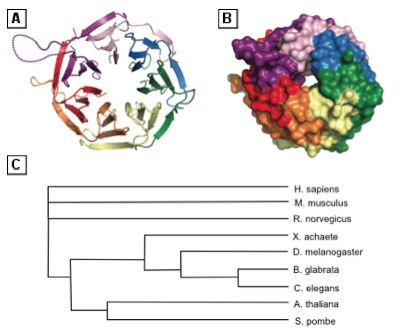
A key component involved in diverse functions
RACK1 function was assessed in many model organisms and has been shown to have different functions. Deletion of RACK1 orthologue in Saccharomyces cerevisiae, Asc1p, is not lethal but leads to a shift towards a hypoxic energy metabolism. Moreover, Asc1p mutants show an increased fermentation and a lack of respiration [13], suggesting a role in metabolism regulation. In the contrary, depletion of RACK1 in pluricellular organisms is lethal. For instance, fruit flies depleted from RACK1 never develop into adults [14]. RACK1 is ubiquitously expressed and required during embryogenesis of Drosophila melanogaster. Moreover, RACK1 shows specific enrichment in the ovary, may play a role in production, partitioning or maintenance of the 16 germ cells in each egg chamber, and is essential for egg maturation. Furthermore, RACK1 analysis in Caenorhabditis elegans showed that it plays a role in germline development and furrow's ingress [15]. It is also required for Xenopus development, since the interaction between RACK1 and Tyrosine Protein kinase-like 7 (PTK7) is required for neural tube closure, suggesting an impact of RACK1 on cell migration [3]••. These datas were confirmed in mice. Indeed, homozygous mutation is lethal at the gastrulation stage but RACK1 heterozygous mutants are viable and have skin pigmentation defects that may be linked to a lack of melanocytes migration. Furthermore, RACK1 expression was associated with proliferation in the early embryonic development of chick limb [2]••.
Besides these roles in cell signaling, it has been shown that in Schizosaccharomyces pombe, Cpc2 (RACK1 orthologue) may control Mitogen-Activated Protein Kinase (MAPK) pathway activation, oxydative stress response and cell cycle progression by translation of specific genes [16]. Moreover, in heterozygous mutant mice, protein synthesis in response to extracellular stimuli as well as PKC-stimulated translation is reduced [17]•, showing that RACK1 acts as an adaptor that converges signaling pathways to the ribosomal machinery. Its involvement in microRNA (miRNA) was also shown in the nematode [18]••.
These results suggest an important role of RACK1 in translation, miRNA function, metabolism, embryogenesis, signaling, proliferation and migration. At the level of organisms, it seems to be a link between RACK1 and cellular functions involved in human cancer. However, the majority of the studies were done in model organisms, but the fact that RACK1 was found to be upregulated in some cancers confirms the possibility of its involvement in cancerogenesis. A switch at the molecular level may help to understand its role in cancer progression.
RACK1, cellular signaling and cancer
Opposed roles in cell growth: proliferation versus cytostasis
RACK1 slows down cell proliferation by an inhibitory effect on Src protein, a tyrosine kinase involved in cell growth and differentiation. As a proto-oncogene, Src misregulation leads to cancer. PKC activation translocate the RACK1/PKC complex to the membrane, where it colocalizes with Src [19]. RACK1 phosphorylation by Src next allows the interaction between both proteins. Moreover, RACK1 inhibits some Src kinase activity, especially on Src Associated in Mitosis 68 (Sam68), a protein involved in cell cycle progression (Figure 2) [20]. This Src inhibition is particularly important at the G1 cell cycle checkpoint because RACK1 overexpression in NIH-3T3 cells induces a partial G1 arrest by inhibition of Myc among others (Figure 2) [21]. In colon cells for instance, RACK1 blocks key cell growth regulators via Src inhibition. Moreover, it prevents mitotic exit by maintaining the Cyclin-Dependent Kinase 1 (CDK1)/Cyclin B complex in an activated state (Figure 2) [5]••. These actions of RACK1 on kinases lead to cytostasis.
In the contrary, RACK1 induces cell proliferation via the MAPK pathway, an important component of cellular environment perception. Three distinct pathways are part of it: Extracellular Regulated Kinase (ERK), Jun N-terminal Kinase (JNK) and p38. ERK is activated by mitogens whereas JNK and p38 are activated by environmental stresses and control proliferation. MAP Kinase Kinase 7 (MKK7), one of the kinases involved in JNK signaling pathway, interacts with RACK1 [22]. This interaction increases MKK7/JNK activity in hepatocellular carcinoma (HCC) cells, in which RACK1 is overexpressed. Moreover, RACK1 may be an adaptor for PKC-mediated JNK activation (Figure 2) [23].
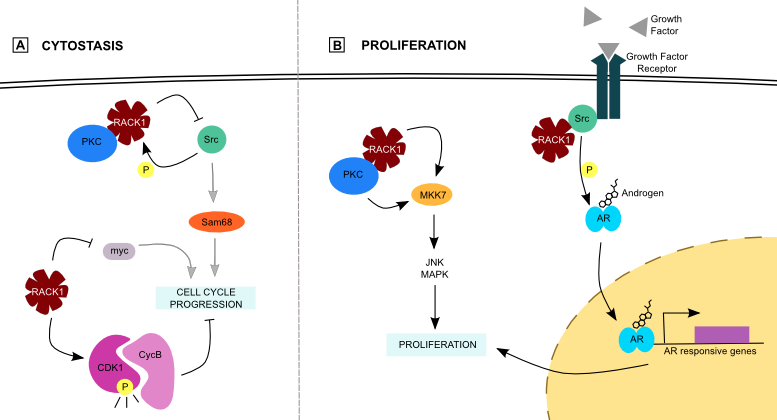
Figure 2. RACK1 and proliferation..
(A) RACK1 prevents cell growth by its inhibition on Src and myc, or by maintenance of an active CDK1/CycB complex that prevents cell cycle progression. (B) On the other side, it enhances proliferation by enhancement of both MKK7 and AR activity.
Modulation of Androgen Receptor (AR) activity is another way to regulate proliferation. AR is a nuclear receptor involved in prostate cancer that activates transcription of genes involved in proliferation and differentiation upon androgen stimulation. The RACK1/Src complex may play a role in AR phosphorylation and activation (Figure 2) [24]. It is possible for RACK1 to mediate androgen and growth factor cross-talk as it facilitates AR phosphorylation. This cross-talk could mediate androgen-independence of those cancer cells. However, the fact that Src is the kinase that phosphorylates AR was not shown in vivo, and one must not forget that RACK1 inhibits some Src functions [20]. If Src is really involved in AR phosphorylation, it should be in a way that is not inhibited by RACK1 binding.
All in all, RACK1 has opposed roles on cell proliferation. On one hand, it leads to cytostasis, mainly through Src inhibition, while on the other hand it induces proliferation through the MAPK pathway or by stimulation of the AR activity.
A versatile role in cell death
Programmed cell death is required during the whole life of any organism. Escape from apoptosis is one of the key features of cancer cells because it leads to uncontrolled cell growth and chemoresistance.
Apoptosis is regulated by a balance between pro- and anti-apoptotic proteins. In the intrinsic pathway, BimEL, a splicing variant of the proapoptotic Bim protein, interacts with the antiapoptotic Bcl-2 protein, leading to Bax freeing. Bax forms pores on the mitochondria, allowing Cytochrome c release, Caspase 3 and 9 activation and cell death. In breast cancer, RACK1 promotes BimEL proteasomal degradation upon apoptotic stimulation, favoring the interaction between Bcl-2 and Bax that prevents cells from apoptosis (Figure 3) [25]. This shows the importance of an equilibrated RACK1 quantity. Furthermore, RACK1 interacts with p73α, a p53 related protein that activates transcription from p53 responsive promoters in response to genotoxic stress and DNA damage [26]. By this interaction, the p73α-mediated transcription of proapoptotic genes like p21 and Bax is inhibited. This effect is antagonized by binding of the tumor suppressor protein Retinoblastoma protein (RB) to RACK1 (Figure 3), suggesting a functional link between p73, RACK1 and RB.
Since the main problem of chemotherapies is resistance to cell death, the question of RACK1-mediated survival is of the utmost interest. A study showed a potential role of RACK1 in the resistance to etoposide [27]••. Some stresses such as hypoxia induce cells to form cytoplasmic stress granules (SGs) which prevent accumulation of misfolded proteins. RACK1 is one of the proteins sequestered in SGs because of its binding to the ribosomes. On the other side, genotoxic drugs and X-rays used in therapeutics activate p38 and JNK pathways, causing cell death. This is facilitated by RACK1 interaction with MAP/ERK Kinase Kinase 4 (MEKK4). Antagonistic effects may thus occur, for instance when both stresses are simultaneous -hypoxia and chemotherapy for instance- because sequestration of RACK1 into SGs suppresses p38 and JNK activation (Figure 3).
Conversely, recent studies tend to give proapoptotic functions to RACK1. Src protects colon cancer cells from apoptosis and RACK1 inhibition of Src promotes mitochondrial cell death and blocks Akt-mediated cell survival (Figure 3). Moreover, RACK1 blocks antiapoptotic Bcl-2 and Bcl-xL expression, and induces proapoptotic Bim expression (Figure 3) [6]. As a consequence, RACK1 participates to the death of colon cells. Furthermore, RACK1 dissociates the Bax/Bcl-xL complex, promoting UV-induced apoptosis. It also facilitates Bax oligomerization, which permeabilizes the mitochondrial membrane and releases apoptotic factors (Figure 3) [28]. This proapoptotic function is still discussed in breast cancer, where two studies found opposed roles for RACK1. One of these identified RACK1 as a marker of poor clinical outcome whereas the other confirmed RACK1 proapoptotic role [9,29].
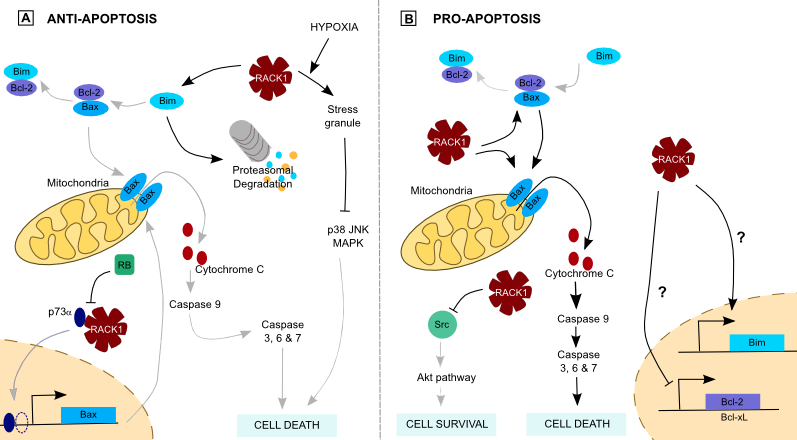
Figure 3. RACK1 and apoptosis..
(A) RACK1 inhibits apoptosis through Bim degradation, preventing pore formation at the mitochondria. It also prevents p73-mediated transcription of the proapoptotic Bax protein. Its sequestration in stress granules takes part in chemotherapy resistance of cancer cells. (B) In contrast, RACK1 may induce apoptosis by dissociating Bax from Bcl-2 and favors Bax oligomerization, leading in cytochrome C release. Moreover, its inhibition of Src prevent Akt-mediated cell survival and RACK1 may induce proapoptotic proteins translation.
To summarize, RACK1 plays differential roles in apoptosis. It acts as a pro- or anti-apoptotic factor, using the same actors but depending on the cellular context (Figure 3). This complexity of biological systems shows the need to consider RACK1 in its cellular environment, with its different target proteins.
RACK1, migration and angiogenesis: a potential role in metastasis
Integrins are heterodimeric αβ receptors for ExtraCellular Matrix (ECM) proteins that are tethering cells to the ECM. RACK1 interacts with the integrin β subunit and may link integrin to PKC, which regulates integrin activity [2]••. Their activation lead to Focal Adhesion Kinase (FAK) phosphorylation, which activates ERK and PI3K/Akt among others, allowing cell migration. In normal cells, loss of integrin signaling leads to anoïkis, a particular programmed cell death, but tumor cells are already cell death resistant. Those pathways may be subverted in cancer cells and loss of tissue integrity is the first step to metastasis.
RACK1 regulates the organization of focal adhesion and the cellular protrusive activity through Src-interaction, and is also required for chemotactic cell migration [30]. In prostate cancer, the transmembrane glycoprotein Trop-2 is upregulated. This protein stimulates β1-integrin and RACK1 association. This lead to Src and FAK activation, ending in ECM detachment. This mechanism may be used by cancer cells to detach and invade the surrounding ECM [31]. RACK1 was previously shown to inhibit Src, but has no effect on the Src-mediated activation of the ERK pathway, which is important for integrin signaling [21]. Moreover, RACK1 is required for active ERK localization to focal adhesion (Figure 4), inducing cell migration [32].
In squamous cancer cells, RACK1 forms a complex at nascent adhesions with FAK and PDE4D5, a c-AMP-degrading phosphodiesterase (Figure 4). This binding is required for chemotactic cancer cell invasion. FAK, RACK1 and PDE4D5 may thus be part of a “direction-sensing” pathway [4]••. FAK has another downstream effector: Rho proteins are part of a pathway which plays a role in actomyosin contractility and mediates tumor cells invasive behavior. RACK1 promotes breast cancer cells migration through Rho kinase pathway (Figure 4) and higher RACK1 expression correlates with shorter survival time [10]. Rho activation could thus be triggered by RACK1 binding to FAK.
Integrin signaling is synergistic with the Insulin-like Growth Factor-I (IGF-I) pathway. RACK1 forms a complex with PKC and IGF-I Receptor (IGF-IR), establishing it as a possible link between IGF-IR and integrin pathway [2]••. RACK1 also binds to Protein Phosphatase 2A (PP2A), a negative regulator of Akt and binding of PP2A or β1-integrin to RACK1 is mutually exclusive (Figure 4) [33]. As RACK1 is involved in integrin-IGF-I pathway activation, the balance of its interaction with PP2A or β1-integrin is part of Akt regulation, which controls migration. In normal conditions, RACK1 binds to activated Ephrin type-B receptor 3 (EphB3), and Akt is thus inhibited by PP2A. In Non-Small-Cell Lung Cancer (NSCLC), ligands of EphB3 are downregulated, leading to a decreased activation of EphB3 receptor and increased Akt activity, promoting metastasis [34]. Moreover, RACK1 regulates Signal Transducers and Activators of Transcription 3 (STAT3) activation by recruiting it to integrin and IGF-IR, bringing STAT3 closer to its activating kinase, a Janus kinase (JAK), which enhances STAT3 transcriptional activity (Figure 4) [35]. As c-myc and vegf are important STAT3 target genes, the role of this interaction in tumorigenesis has to be taken into account.
Another actor regulating IGF-IR liaison with RACK1 is the Von Hippel Lindau (VHL) protein, an E3 ubiquitin ligase which loss is involved in renal carcinoma. VHL competes with IGF-IR for RACK1 binding and its downregulation leads to an increased RACK1 binding to IGF-IR, and to invasive potential [36]. Moreover, VHL degrades Hypoxia Induced Factor-1α (HIF-1α), a subunit of HIF transcription factor that plays a role in angiogenesis. RACK1 competes with Heat-Shock Protein 90 (HSP90) for HIF-1α binding (Figure 4) [37]. HSP90 is a molecular chaperone that protects HIF-1α from degradation. Consequently, RACK1 promotes HIF-1α degradation independently of O2 and VHL by Elongin-C binding and is supposed to be a major determinant of the basal HIF-1α degradation rate. RACK1 deregulation could thus give rise to an increased blood vessels formation. This is confirmed by the fact that RACK1 has been found to be upregulated during angiogenesis [3]••.
In cardiomyocytes, RACK1 influences migration, but doesn't associate with IGF-IR. It modulates it by its impact on ERK signaling (Figure 4) [38]. This study points out that cell type must be taken into account as RACK1 is concerned. Altered competition for binding sites on RACK1 may thus distinguish normal from tumor cells.
Altogether, these data suggest that RACK1 promotes loss of ECM adhesion and tumor cells migration, mainly by its involvement in integrin signaling pathway. Conversely, it stimulates E-cadherin-mediated cell-cell junctions. These proteins undergo Src- or growth factor- induced endocytosis that is inhibited in colon cells by RACK1-mediated Src inhibition (Figure 4) [7]•. RACK1 has thus the potential to reduce invasive properties of colon carcinoma cells by E-cadherin endocytosis regulation.
Consequently, RACK1 plays a versatile role in migration, and may also take part in angiogenesis. Such involvements suggest a role for RACK1 in metastasis.
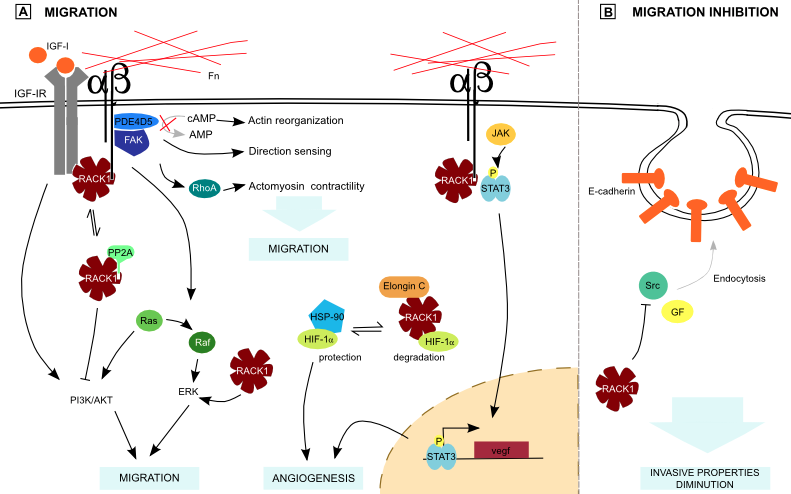
Figure 4. RACK1 and metastasis..
(A) RACK1 forms a complex with PDE4D5 and FAK downstream of integrin signaling, favoring cell migration. Moreover, it stimulates Rho proteins. Independently of those signaling pathways, RACK1 enhances ERK activity. PP2A and integrin β competition for RACK1 binding plays a role in PI3K/Akt regulation. RACK1 also competes with HSP-90 for HIF-1α binding, what participates in angiogenesis regulation. Binding of RACK1 with integrin β and STAT3 brings it closer to its activating kinase JAK, favoring angiogenesis. All those interactions are part of ECM adhesion loss. Fn: Fibronectin. (B) Otherwise, RACK1 stimulates cell-cell adhesion by preventing E-cadherin endocytosis thanks to Src inhibition.
Ribosomal function of RACK1
Selectivity of mRNA translation
Rack1 is a ribosomal protein and, as such, its translation depends on serum and amino acids availability [39]. Consequently, its ribosomal function must be considered in tumorigenesis. RACK1 strongly binds to the 40S ribosomal subunit (Figure 5), near to the mRNA exit channel. Because of its strong binding, the presence of cytosolic RACK1 may be due to an overexpression rather than to ribosome dissociation. RACK1 might thus assemble signaling complexes at the ribosome and allow translation regulation in response to cell stimuli [40]•. As Asc1p mutants show elevated levels of some proteins, one of Asc1p functions may be gene repression [41]. Moreover, RACK1 plays a role in translation selectivity. In HCC, coupling between RACK1 and PKCβII promotes eIF4E phosphorylation and leads to preferential translation of Survivin and Bcl-2 (Figure 5), showing the link between RACK1 ribosomal function and apoptosis inhibition [8]. Likewise, Scp160p, a member of the Vigilin RNA-binding proteins family, requires RACK1 interaction for ribosome binding [40]•. This interaction might regulate specific mRNA translation (Figure 5).
RACK1 is involved in eIF6 release from the 60S ribosomal subunit through PKCβII, allowing subunits joining and translation (Figure 5). RACK1 provides thus a physical and functional link between PKC and ribosome activation [42]. Ribosomes host active PKC, which phosphorylates ribosome-associated substrates like initiation factors and mRNA-binding proteins. Translocation of PKCβII to ribosomes increases the rate of translation [43]. As a result, RACK1 deregulation could give rise to an uncontrolled ribosomal activity through PKC overactivation. This control of gene expression may also be performed at the elongation level because RACK1 participates in peptide-dependent translation arrest and leads to endonucleocytic cleavage and degradation of specific mRNA (Figure 5) [44]. RACK1 has thus a role in the control of gene expression.
Using mass spectrometry, RACK1 was identified in Internal Ribosome Entry Site (IRES)-associated initiation complex [45]. This is not surprising because RACK1 has been shown to be a ribosomal protein. But this could indicate a role for RACK1 in IRES-mRNA translation too (Figure 5). The potential link with IRES-containing mRNA raises an interesting possibility in cancer genes regulation. Namely, some genes as c-myc, vegf and hif-1α mRNAs are IRES-dependent. Accordingly, RACK1 may play a role in their upregulation during cancer progression.
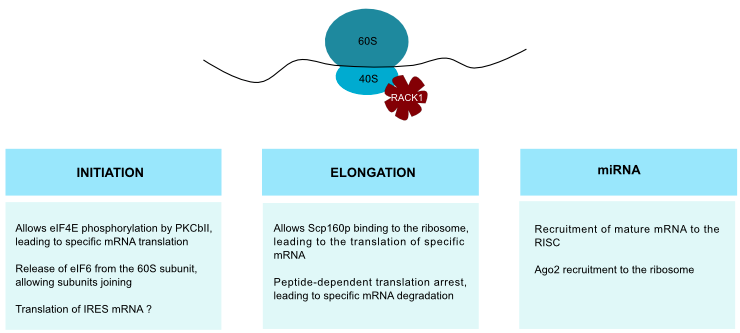
Figure 5. RACK1 and ribosome. .
Thanks to its interaction with the ribosome, RACK1 plays various role in ribosomal function as modulation of the first steps of translation and miRNA efficiency.
miRNA function: ribosomal recruitment to target sites
Recent studies showed that RACK1 takes part in the miRNA pathway. Those single stranded small RNAs regulate gene expression by inducing degradation of target mRNAs. They seem to be involved in cancer progression, since variations in miRNA levels is a general trait of human cancers. It was shown that RACK1 links to KH-type Splicing Regulatory Protein (KSRP), a protein that is part of the DICER complex that processes long dsRNA into small RNAs [46]. This complex recruits mature miRNAs to Ago protein, forming the miRISC (RNA-Induced Silencing Complex) that mediates post-transcriptional gene silencing. RACK1 is involved in the recruitment of miRNAs into miRISC (Figure 5) [18]••. Its expression is reduced in HCC, and consequently miRNA function is impaired too. RACK1 contributes to Ago2 recruitment to the ribosome (Figure 5) and may facilitate interaction between component of RISC and translational machinery.
To summarize, RACK1 is a ribosomal protein that plays a role in translational selectivity and miRNA function. Deregulation of any of those processes can lead to tumorigenesis.
Conclusion and perspectives
RACK1 plays a role in key cancerous processes as proliferation, apoptosis, migration and angiogenesis. Its opposite functions may be due to different binding partners, deferring among cell type. Moreover, RACK1 is a ribosomal protein that allows translation selectivity and miRNA pathway efficiency. Both functions are part of tumorigenesis. Focus was made on RACK1 function in disease, but one must keep in mind that RACK1 has above all physiological functions, and that RACK1 deregulation may participate in cancerogenesis. Some studies proposed to use RACK1 as a biomarker, others even suggested that it could be used as a target in therapy. But for now, its complete role in a single cell is not well understood. Its targeting would be complicated because of unsuspected side effects, showing the need to better understand RACK1 networks. In some cases, RACK1 acts by protein sequestration or by competition to regulate target protein activation. A tight regulation of RACK1 quantity is required because RACK1 may act as a balance between signals, thus its deregulation has major consequences for homeostasis. In addition to those differential functions, it is of first interest to note that RACK1 downregulation has various effects among species. Consequently, RACK1 may have many potential functions rather than a defined one.
A striking observation that can be made with RACK1's scientific literature is the gap between ribosome and signaling research. Both roles of RACK1 are rarely studied together, which would be required to get an integrated overview of its function. Besides, studies on yeast only show a ribosomal function for RACK1 and even if RACK1 was shown to control MAPK pathway in fission yeast, it was by regulation of translation. RACK1 may thus primarily function as a translational regulator. In multicellular organisms, it is consequently possible that RACK1 had acquired new functions in direct signaling regulation, mainly because of its structure that facilitates interactions. However, it is possible that this lack of information on yeast signaling regulation comes from an absence of such studies. Study of RACK1 is thus a very complex, promising and exciting field of research.
Abbreviations
AR: Androgen Receptor; ECM: Extracellular Matrix; ERK: Extracellular Regulated Kinase; FAK: Focal Adhesion Kinase; HCC: Hepatocellular Carcinoma; HIF: Hypoxia Inducible Factor; IGF-IR: Insulin-like Growth Factor-I Receptor; JNK: Jun N-terminal Kinase; MAPK: Mitogen-Activated Protein Kinase; miRNA: micro-RNA; PKC: Protein Kinase C; RACK1: Receptor for Activated Protein C Kinase 1.
Acknowledgments
I would like to thank Mrs Magali Naville and Dr Jean-Luc Imler for their advices concerning this review, as well as Mr Alexandre Cammarata for his comments on the figures.
References and recommended reading
Papers of particular interest have been highlighted as:
● of special interest
●● of outstanding interest
1.
Hanahan D, Weinberg RA: Hallmarks of Cancer: The Next Generation. Cell 2011, 144:646–674.
[Full Paper] ●● Global review that try to provide a framework for
understanding neoplastic diseases. Ten hallmarks of cancer cells are
described, allowing a global understanding of this disease.
2. McCahill A, Warwicker J, Bolger
GB, Houslay MD, Yarwood SJ: The RACK1 scaffold protein: a dynamic
cog in cell response mechanisms. Mol Pharmacol 2002,
62:1261–1273. [Full
Paper] ●● Review that puts the basis of RACK1's structure, protein interaction,
signal transduction, and role in physiological functions. It helps to gain a
precise knowledge on RACK1 research.
3.
Adams DR, Ron D, Kiely PA: RACK1, A multifaceted scaffolding protein: Structure and
function. Cell Commun. Signal 2011, 9:22. [Full Paper][PubMed] ●● This recent review collects
recent studies on the role of RACK1 in physiological processes as
development, cell migration, nervous system function and circadian rhythm,
and how it can be subverted in diseases.
4.
Gibson TJ: RACK1
research - ships passing in the night?FEBS
Lett. 2012, 586:2787–2789. [Full Paper][PubMed] ● General short
review about RACK1 that puts down concepts of RACK1 research and the
question of RACK1's role in signaling pathways and
ribosome.
5.
Mamidipudi V, Dhillon NK, Parman T, Miller LD, Lee KC, Cartwright CA:
RACK1 inhibits colonic cell growth
by regulating Src activity at cell cycle checkpoints. Oncogene 2007,
26:2914–2924. [Full Paper] ● This study of RACK1 and Src interaction in colon
cells shows a new mechanism of how cell cycle control can be performed by
RACK1 on G1 phase and mitotic checkpoints.
6. Mamidipudi V, Cartwright CA: A novel pro-apoptotic function of RACK1: suppression of Src activity in the intrinsic and Akt pathways. Oncogene 2009, 28:4421–4433. [Full Paper]
7. Swaminathan G, Cartwright CA:
Rack1 promotes epithelial cell–cell adhesion by regulating
E-cadherin endocytosis. Oncogene 2011, 31:376–389. [Full
Paper] ● This study shows that RACK1 promotes cell-cell adhesion via Src
inhibition in colon cancer cells. Its role in the regulation of E-cadherin
endocytosis suggests it maintains the junctional homeostasis of intestinal
epithelial cells.
8. Ruan Y, Sun L, Hao Y, Wang L, Xu J, Zhang W, Xie J, Guo L, Zhou L, Yun X, et al.: Ribosomal RACK1 promotes chemoresistance and growth in human hepatocellular carcinoma. J. Clin. Invest. 2012, 122:2554–2566. [Full Paper][PubMed]
9. Cao X-X, Xu J-D, Liu X-L, Xu J-W, Wang W-J, Li Q-Q, Chen Q, Xu Z-D, Liu X-P: RACK1: A superior independent predictor for poor clinical outcome in breast cancer. International Journal of Cancer 2010, 127:1172–1179. [Full Paper]
10. Cao X-X, Xu J-D, Xu J-W, Liu X-L, Cheng Y-Y, Li Q-Q, Xu Z-D, Liu X-P: RACK1 promotes breast carcinoma migration/metastasis via activation of the RhoA/Rho kinase pathway. Breast Cancer Research and Treatment 2010, 126:555–563. [Full Paper]
11. Sengupta J, Nilsson J, Gursky R, Spahn CMT, Nissen P, Frank J: Identification of the versatile scaffold protein RACK1 on the eukaryotic ribosome by cryo-EM. Nature Structural & Molecular Biology 2004, 11:957–962. [Full Paper]
12. Wang S, Chen J-Z, Zhang Z, Gu S, Ji C, Tang R, Ying K, Xie Y, Mao Y: Cloning, expression and genomic structure of a novel human GNB2L1 gene, which encodes a receptor of activated protein kinase C (RACK). Mol. Biol. Rep. 2003, 30:53–60. [PubMed]
13. Rachfall N, Schmitt K, Bandau S, Smolinski N, Ehrenreich A, Valerius O, Braus GH: RACK1/Asc1p, a ribosomal node in cellular signaling. Mol. Cell Proteomics 2012, doi:10.1074/mcp.M112.017277. [Full Paper][PubMed]
14. Kadrmas JL, Smith MA, Pronovost SM, Beckerle MC: Characterization of RACK1 function in Drosophila development. Dev. Dyn. 2007, 236:2207–2215. [Full Paper][PubMed]
15. Skop AR, Liu H, Yates J 3rd, Meyer BJ, Heald R: Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science 2004, 305:61–66. [Full Paper][PubMed]
16. Núñez A, Franco A, Madrid M, Soto T, Vicente J, Gacto M, Cansado J: Role for RACK1 orthologue Cpc2 in the modulation of stress response in fission yeast. Mol. Biol. Cell 2009, 20:3996–4009. [Full Paper][PubMed]
17.
Volta V, Beugnet A, Gallo S, Magri L, Brina D, Pesce E, Calamita P,
Sanvito F, Biffo S: RACK1 depletion
in a mouse model causes lethality, pigmentation deficits and reduction in
protein synthesis efficiency.
Cell. Mol. Life
Sci. 2012,
doi:10.1007/s00018-012-1215-y. [Full Paper][PubMed] ● The authors produced the first RACK1 mutant mouse,
allowing them to study deficient embryos and adult mice.
18. Jannot G, Bajan S, Giguère NJ,
Bouasker S, Banville IH, Piquet S, Hutvagner G, Simard MJ: The
ribosomal protein RACK1 is required for microRNA function in both C. elegans and humans. EMBO
Rep. 2011, 12:581–586. [Full
Paper][PubMed] ●● This study demonstrates the direct interaction between miRISC and RACK1,
suggesting that RACK1 contributes to the recruitment of miRISC to the
translation site.
19. Chang BY, Harte RA, Cartwright CA: RACK1: a novel substrate for the Src protein-tyrosine kinase. Oncogene 2002, 21:7619–7629. [Full Paper][PubMed]
20. Miller LD, Lee KC, Mochly-Rosen D, Cartwright CA: RACK1 regulates Src-mediated Sam68 and p190RhoGAP signaling. Oncogene 2004, 23:5682–5686. [Full Paper][PubMed]
21. Mamidipudi V, Zhang J, Lee KC, Cartwright CA: RACK1 regulates G1/S progression by suppressing Src kinase activity. Mol. Cell. Biol. 2004, 24:6788–6798. [Full Paper][PubMed]
22. Guo Y, Wang W, Wang J, Feng J, Wang Q, Jin J, Lv M, Li X, Li Y, Ma Y, et al.: Receptor for activated C kinase 1 promotes hepatocellular carcinoma growth by enhancing mitogen-activated protein kinase kinase 7 activity. Hepatology 2012, doi:10.1002/hep.25978. [Full Paper][PubMed]
23. Lopez-Bergami P, Habelhah H, Bhoumik A, Zhang W, Wang L-H, Ronai Z: RACK1 mediates activation of JNK by protein kinase C [corrected]. Mol. Cell 2005, 19:309–320. [Full Paper][PubMed]
24. Kraus S, Gioeli D, Vomastek T, Gordon V, Weber MJ: Receptor for Activated C Kinase 1 (RACK1) and Src Regulate the Tyrosine Phosphorylation and Function of the Androgen Receptor. Cancer Res 2006, 66:11047–11054. [Full Paper]
25. Zhang W, Cheng GZ, Gong J, Hermanto U, Zong CS, Chan J, Cheng JQ, Wang L-H: RACK1 and CIS mediate the degradation of BimEL in cancer cells. J. Biol. Chem. 2008, 283:16416–16426. [Full Paper][PubMed]
26. Ozaki T, Watanabe K, Nakagawa T, Miyazaki K, Takahashi M, Nakagawara A: Function of p73, not of p53, is inhibited by the physical interaction with RACK1 and its inhibitory effect is counteracted by pRB. Oncogene 2003, 22:3231–3242. [Full Paper][PubMed]
27.
Arimoto K, Fukuda H, Imajoh-Ohmi S, Saito H, Takekawa M:
Formation of stress granules
inhibits apoptosis by suppressing stress-responsive MAPK
pathways. Nature Cell Biology 2008, 10:1324–1332. [Full Paper] ●● By analyzing the role of RACK1 in MTK1 activation,
the authors found that RACK1 functions as a mediator between hypoxia and
genotoxic drugs response. These findings may explain the reason of
hypoxia-induced resistance to cancer therapy.
28. Wu Y, Wang Y, Sun Y, Zhang L, Wang D, Ren F, Chang D, Chang Z, Jia B: RACK1 promotes Bax oligomerization and dissociates the interaction of Bax and Bcl-XL. Cell. Signal. 2010, 22:1495–1501. [Full Paper][PubMed]
29. Al-Reefy S, Osman H, Jiang W, Mokbel K: Evidence for a pro-apoptotic function of RACK1 in human breast cancer. Oncogene 2010, 29:5651–5651. [Full Paper]
30. Cox EA, Bennin D, Doan AT, O'Toole T, Huttenlocher A: RACK1 regulates integrin-mediated adhesion, protrusion, and chemotactic cell migration via its Src-binding site. Mol. Biol. Cell 2003, 14:658–669. [Full Paper][PubMed]
31. Trerotola M, Li J, Alberti S, Languino LR: Trop-2 inhibits prostate cancer cell adhesion to fibronectin through the beta(1) integrin-RACK1 axis. J. Cell. Physiol. 2012, 227:3670–3677. [Full Paper]
32. Vomastek T, Iwanicki MP, Schaeffer H-J, Tarcsafalvi A, Parsons JT, Weber MJ: RACK1 targets the extracellular signal-regulated kinase/mitogen-activated protein kinase pathway to link integrin engagement with focal adhesion disassembly and cell motility. Mol. Cell. Biol. 2007, 27:8296–8305. [Full Paper][PubMed]
33. Kiely PA, O'Gorman D, Luong K, Ron D, O'Connor R: Insulin-like growth factor I controls a mutually exclusive association of RACK1 with protein phosphatase 2A and beta1 integrin to promote cell migration. Mol. Cell. Biol. 2006, 26:4041–4051. [Full Paper][PubMed]
34. Li G, Ji X-D, Gao H, Zhao J-S, Xu J-F, Sun Z-J, Deng Y-Z, Shi S, Feng Y-X, Zhu Y-Q, et al.: EphB3 suppresses non-small-cell lung cancer metastasis via a PP2A/RACK1/Akt signalling complex. Nat Commun 2012, 3:667. [doi: 10.1038/ncomms1675] [PubMed]
35. Zhang W, Zong CS, Hermanto U, Lopez-Bergami P, Ronai Z, Wang L-H: RACK1 recruits STAT3 specifically to insulin and insulin-like growth factor 1 receptors for activation, which is important for regulating anchorage-independent growth. Mol. Cell. Biol. 2006, 26:413–424. [Full Paper][PubMed]
36. He X, Wang J, Messing EM, Wu G: Regulation of receptor for activated C kinase 1 protein by the von Hippel-Lindau tumor suppressor in IGF-I-induced renal carcinoma cell invasiveness. Oncogene 2011, 30:535–547. [Full Paper][PubMed]
37. Liu YV, Baek JH, Zhang H, Diez R, Cole RN, Semenza GL: RACK1 competes with HSP90 for binding to HIF-1alpha and is required for O(2)-independent and HSP90 inhibitor-induced degradation of HIF-1alpha. Mol. Cell 2007, 25:207–217. [Full Paper][PubMed]
38. O'Donovan HC, Kiely PA, O'Connor R: Effects of RACK1 on cell migration and IGF-I signalling in cardiomyoctes are not dependent on an association with the IGF-IR. Cell. Signal. 2007, 19:2588–2595. [Full Paper][PubMed]
39. Loreni F, Iadevaia V, Tino E, Caldarola S, Amaldi F: RACK1 mRNA translation is regulated via a rapamycin-sensitive pathway and coordinated with ribosomal protein synthesis. FEBS Lett. 2005, 579:5517–5520. [Full Paper][PubMed]
40.
Nilsson J, Sengupta J, Frank J, Nissen P: Regulation of eukaryotic translation by the RACK1 protein:
a platform for signalling molecules on the ribosome. EMBO
Rep. 2004, 5:1137–1141.
[Full Paper][PubMed] ● This review mainly emphasize the role of RACK1 as
a ribosomal protein and discuss the way it may regulate
translation.
41. Gerbasi VR, Weaver CM, Hill S, Friedman DB, Link AJ: Yeast Asc1p and mammalian RACK1 are functionally orthologous core 40S ribosomal proteins that repress gene expression. Mol. Cell. Biol. 2004, 24:8276–8287. [Full Paper][PubMed]
42. Ceci M, Gaviraghi C, Gorrini C, Sala LA, Offenhäuser N, Marchisio PC, Biffo S: Release of eIF6 (p27BBP) from the 60S subunit allows 80S ribosome assembly. Nature 2003, 426:579–584. [Full Paper]
43. Grosso S, Volta V, Vietri M, Gorrini C, Marchisio PC, Biffo S: Eukaryotic ribosomes host PKC activity. Biochem. Biophys. Res. Commun. 2008, 376:65–69. [Full Paper][PubMed]
44. Kuroha K, Akamatsu M, Dimitrova L, Ito T, Kato Y, Shirahige K, Inada T: Receptor for activated C kinase 1 stimulates nascent polypeptide-dependent translation arrest. EMBO Rep. 2010, 11:956–961. [Full Paper][PubMed]
45. Yu Y, Ji H, Doudna JA, Leary JA: Mass spectrometric analysis of the human 40S ribosomal subunit: Native and HCV IRES-bound complexes. Protein Science 2009, 14:1438–1446. [Full Paper]
46. Otsuka M, Takata A, Yoshikawa T, Kojima K, Kishikawa T, Shibata C, Takekawa M, Yoshida H, Omata M, Koike K: Receptor for activated protein kinase C: requirement for efficient microRNA function and reduced expression in hepatocellular carcinoma. PLoS ONE 2011, 6:e24359. [Full Paper][PubMed]


