Evidence for a link between early life stress and adult aggression – The role of the hypothalamus-pituitary-adrenal axis
2013/01/21
Abstract
Early-life stress (ELS) is told to participate in the emergence of many psychopathologies during adulthood such as PTSD or depression. In order to understand better the consequences of an ELS in the adulthood, many models have been established. One of the main models used in different species is the separation of the child from his mother during childhood. The hypothalamo-pituitary-adrenal (HPA) axis is the main axis regulating the stress response in Mammals. All studies agree in underlying that the components of this axis are altered after an ELS. Furthermore, different studies in rodents as in primates have shown that the different components of the HPA axis, such as the vasopressin, may play an important role in the emergency of aggression in a sex-specific manner. Thus, it is essential to understand how the alterations of the HPA axis induced by an ELS, such as repeated maternal separation, may induce alterations in aggression in adulthood.
Table of Contents
- Introduction
- On what period of the HPA axis development does this model have an impact?
- What are the consequences of the ELS on the child's HPA axis? How is the HPA axis altered in the adult who experienced an ELS?
- How do those ELS-induced modifications of the HPA axis impact on aggression?
- Conclusion
- Acknowledgments
- References and recommended reading
Introduction
Early life stress (ELS) corresponds to a wide range of events which can happen during early childhood: child abuse, neglect and trauma, parental loss due to a death or a divorce or also consumption of drugs during pregnancy. Because of this variety, many models have been used as well in terms of animals: rhesus macaques, common squirrels, rats, mice, guinea pigs, hamsters, as in terms of type of ELS: repeated or definitive mother-infant separation (MS) at different periods of the development, early post-weaning social isolation, post-weaning social subjugation. All kinds of ELS seem to alter the hypothalamo-pituitary-adrenal (HPA) axis and adult aggression. There are different kinds of aggression. As reported by Veenema [1], the main types of adult aggression are: offensive aggression when competing for or protecting resources like food, territory and mating, defensive aggression when the animal is attacked by a conspecific or a predator, predatory aggression for predators when attacking a prey and maternal aggression for the mother protecting her children against an intruder. Each type of aggression differently interacts with the HPA axis and is thus differentially affected by an ELS. Stress is a characteristic reaction linked to something potentially dangerous for the organism. Stress can be whether psychological (fear) or physiological (hypoglycemia, blood loss) or both (lack of mother).
Many studies report a direct link between the development of the HPA axis and ELS. Fewer focus on the link between this phenomenon and adult aggression. Firstly, we will see when ELS affects the HPA axis development. Then we will characterize the consequences of ELS on the child's and on the adult's HPA axis. Finally we will study how these alterations can affect the different kinds of adult aggression. To do so, we will focus on the main models of ELS studied which are rhesus macaques, rats and mice after a separation from the mother during early childhood. Early-life corresponds to the period going from fecundation to the end of puberty. Here, we will only consider it as the period going from birth until puberty, in the frame of this model of MS. This model mimics early parental loss or neglect of the child.
On what period of the HPA axis development does this model have an impact?
How does the HPA axis normally develops in the different animals studied?
The HPA axis consists in the paraventricular nucleus of the hypothalamus, the anterior part of the pituitary gland and the adrenal cortex (Figure 1.A). The hypothalamus and the pituitary gland mostly develop during prenatal stages of the development [2]. The adrenal gland begins its development prenatally but it still needs some time postnatally until it is fully developed. The adrenal cortex ends its maturation between 10 and 20 years in humans and at post-natal day (PND) 35 in mice [3]. Very interestingly, in rodents, the hippocampus, a major factor interacting with the HPA axis (Figure 1), develops from PND 1 to 21 [4]. In primates, it develops in the first years of life. Thus, an ELS during childhood might influence the development of the hippocampus and of the adrenal gland.
In rodents, a certain period named as the stress hyporesponsive period (SHRP) is necessary for the normal development of the brain after birth. It corresponds to a period when a stressor which would normally involve a physiological stress response (increase of glucocorticoids and adrenaline, rise of the cardiovascular tone, modifications of the immune system) has no effect. This period lasts from PND 2 to 12 in mice and from PND 4 to 14 in rats [5], PND 1 being the date of birth. It is mediated through maternal physical care. Indeed, it has been proved that depriving the pups from their mother can disrupt the SHRP [5]. Using a brush on the pups to imitate the maternal care can partly reverse this effect [6–8] while letting the pups see and smell their mother without physical contact does not. Maternal care effects in rodents come from arched-back nursing, licking and grooming [5–8]. Further studies remain to be done on primates to see whether the SHRP is a conserved process among species or if it is specific to rodents. An old study [9] suggests that there is a SHRP in humans from 12 month old and on. Nevertheless, they have not determined how long it lasts.
The normal adult HPA is regulated by the circadian rhythm (Figure 1. A,C) and by the environment (Figure 1. A,B), especially by stressful events, whether they are physiological and/or psychological (see Figure 1 to look at all the components). The end-products of the HPA axis are the glucocorticoids (GCs). In rodents, the principal GC is the corticosterone while in humans and rhesus monkeys, it is the cortisol. GCs mediate the stress response and a negative feedback (Figure 1). The negative feedback aim is to stop the stress response once the stressor is gone.
The HPA axis develops partly prenatally and partly postnatally during the first weeks/years of life. In rodents, a SHRP is necessary for the postnatal development of the axis, but it has still not been identified in primates. MS might influence the postnatal development of the hippocampus and of the adrenal cortex.
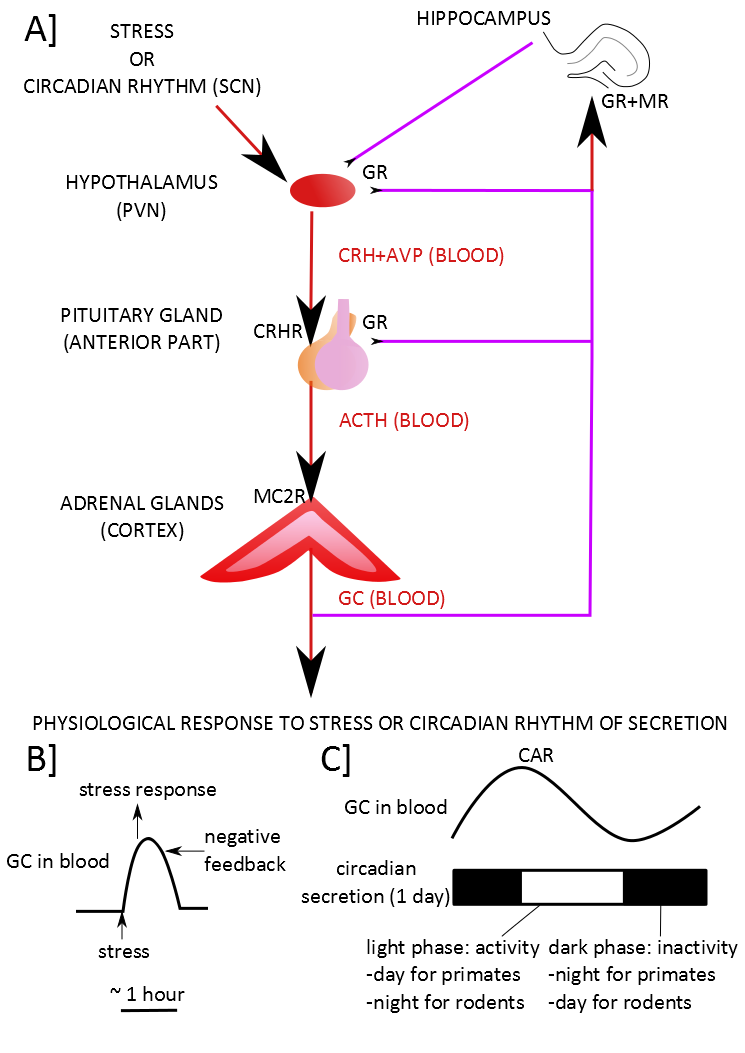
Figure 1. The normal functioning of the HPA axis. .
A] An external stressor or the influence of the projections coming from the suprachiasmatic nucleus (SCN) which regulates the circadian rhythm induces the expression and the release of the cortico-releasing hormone (CRH) and the vasopressin (AVP) by the paraventricular nucleus (PVN) into the portal blood supply of the anterior pituitary gland. The CRH binds to the CRH receptors (CRHR) in the anterior pituitary gland. In the anterior pituitary gland, the AVP enhances the action of CRH. The CRH, when binding to the CRHR, induces the synthesis and the release of the adrenocorticotropic hormone (ACTH) in the blood. ACTH binds to the melanocortin type II receptors (MC2R) in the cortex of the adrenal gland. This binding induces the synthesis and the release of corticoids in the blood. The corticoids induce some physiological modifications. They also activate the negative feedback of the HPA axis directly by inhibiting the secretion of the pituitary gland and the PVN and indirectly by activating some neurons of the hippocampus which will then inhibit the PVN. B] The corticoids induce the physiological response to the stress in response to a stressor. C] Otherwise, they participate to the circadian rhythm. Corticoids act through two types of receptors: the glucocorticoid receptors (GR) and the mineralocorticoid receptors (MR). Red arrows indicate a stimulating input while purple lines indicate an inhibiting input. The CAR is the Cortisol Awakening Response.
What are the different models of MS studied?
As there are so many types of ELS and as much models representing them, we chose here to focus on the main model studied: repeated MS during the SHRP in rats and mice and definitive or repeated separation just after birth in rhesus monkeys. In humans, this could correspond to maternal or parental loss due to an accident or a divorce or to a parental neglect of the child.
It is first essential to pinpoint the slight differences of the protocols between the different models so as to understand the further differences which can appear. Generally, in rodents, the MS consists in taking the dam away from her pups for 3 hours every day, generally from 9am to 12pm, during the SHRP and sometimes a little further of the SHRP. Meanwhile, the pups are handled in an other cage all together with an incubator imitating the mother's heat. This protocol is quite respected among the different studies. It partly comes from the article of Huot et al. [10]. Some test longer or shorter time of separation at different periods (see [5] for review). Sometimes also, pups are left in their homecage all together or handled and separated from each other. It has been reported that this slightly different protocols may affect the pups in a different manner [11]. However, the problem remains on the control used. The results might be quite different whether the control used is undisturbed pups remaining all the time with their mother (non-handled) or just a little handled to clean the cage once a week (animal facility rearing) or pups handled from their mother for only 15 minutes every day. The last one might be closer to the wilderness where the mother might have to let her pups to search for food [5]. On the other side, there might be a bias as the interaction between the mother and pups is modified by the experimenter. In rhesus macaques, the models used do not obey to a generally accepted protocol as it is in rodents. Thus, the protocols are very different. Some try repeated MS similar to those used in rodents [12,13], but most use whether nursery-reared rhesus macaques versus mother-reared [14,15] or peer-reared without their mother versus mother-reared [16]. Consequently, it is interesting to notice that each variation of protocol might have very distinct effects on the HPA axis and aggression.
What are the consequences of the ELS on the child's HPA axis? How is the HPA axis altered in the adult who experienced an ELS?
What are the consequences of MS on the HPA development?
In rodents, repeated MS made during the SHRP disrupts it. The HPA axis of pups which would normally underreact to any stressor is hyperactivated in response to the maternal absence (Table 1). As the SHRP is a critical period for a good maturation of the HPA axis, this implies long-lasting changes in its components. The most important change observed does not affect directly the HPA axis but the hippocampus which is essential for a negative feedback of the axis. It is still developing during the period of MS [4]. Many studies in rodents report that MS during the SHRP as low-caring mothers induce a methylation of the Glucocorticoid Receptor (GR) promoter in the exon 17 in the hippocampus [4,7,17–19]. This leads to low levels of expression of the GR in adulthood. Interestingly, in humans also, child neglect [17], child abuse [20] and prenatal maternal depression [21] are associated with a methylation of the exon 1F of the GR promoter in the hippocampus. The exon 1F is the homologous of the rodent exon 17. ELS are also often related to smaller hippocampus volume in rodents as in humans [22] and to problems in memory related to hippocampal dysfunction [23]. Lajud et al. [24] also showed that MS reduces neurogenesis in the hippocampus during the SHRP. Daniels et al. [25] have shown that at PND21, Sprague Dawley rats have no increase on the methylation of the exon 17 after MS. This might be due to the use of a different strain of rats in front of most studies which use whether Long Evans or Wistar rats or to the fact that at PND21, rats are still young and that the changes might occur lately at adulthood, as most studies of GR promoter are made later on. One last possibility may also rely on the fact that, as it is a repeated MS and not a definitive one, the dam may compensate the absence of her pups by over-caring for them when they are present [26]. Weaver [18] describes more precisely what could be the mechanisms linking maternal care and epigenetic modifications of the GR exon. Interestingly, Huot et al. showed in 2004 [27] that, if the dam was set with another litter during the MS, the effects of the MS on the HPA axis of the pups seemed to be reversed. Sadly, there have been no further research to understand how the maternal behavior was affected by MS and how it could explain the consequences of MS in pups.
All studies agree in underlying that an ELS, here MS, induces alterations in the development of the hippocampus which is essential for the negative feedback of the HPA axis.
What are the consequences of MS on the adult HPA?
There are two principal aspects of the adult HPA which can be affected: the basal activity (circadian rhythm) (Figure 1.C) and the stress-induced activity (Figure 1.B). It appears that MS might affect both aspects on most of the models studied, although there is still much discussion.
In rodents, most of the studies show that MS induces a basal and a stress-induced hyperactivation of the HPA axis, both in male and female adults (Table 1). Nevertheless, some studies also suggest that MS induces no differences of basal nor stress-induced HPA axis activation or even a reduced HPA axis in males [28]. In front of the huge number of studies underlying the hyperactivation of the HPA axis in rodents after MS, it is hard to believe that MS won't induce any effect, although differences in genotypes or in protocols might justify such differences. As shown in Table 1, sometimes the same studies provide different results. This is generally attributed to differences in genotypes, especially in rhesus monkeys [15,16]. Furthermore, many studies do not separate males and females, although there is evidence that the effects of MS are sex-specific in all species (Table 1, [13,28–30]). Others do not provide the age of the animal when the measures are done, although it is known that age is an important factor in HPA axis functioning in Mammals. Especially in males, basal GCs increase with age [31,32]. In addition to that, in females, the HPA axis activity changes with the estrous cycle (Box 1). MS effects may be visible only at a certain period [33], what makes studies in females far more complicated.
Table 1. Consequences of the MS on the basal and on the stress-induced HPA axis activity.
Basal HPA axis activity | Stress-induced HPA axis activity | ||||||||||
Males | Females | Males | Females | ||||||||
young | adult | young | adult | young | adult | young | adult | ||||
Rats | ND | CRH+[1]/~[28], AVP+[57]/-[28], ACTH+[4,51], GC+[1,4,24,51], GRh-[4] | ND | ||||||||
HPA axis | ~ | +/~ | + | + | + | +/~ | |||||
Mice | ND | ND | AVP~[29] | ND | ND | ||||||
HPA axis | + | ND | + | +/~ | |||||||
Rhesus | ND | ND | ND | ND | ND | ||||||
HPA axis | + | ~ | +/~ | ||||||||
Humans | ND | ND | ND | ND | |||||||
HPA axis | +/~ | + | + | ~ | |||||||
ND is for No Data found. + is for an increase of HPA axis activity after MS in front of the control. ~ stands when there is no significant difference with control. - is for a decrease of the HPA axis activity after a MS. Each component of the HPA axis was observed: CRH and AVP released by the PVN, CRHR in the pituitary gland, ACTH and GC released in the blood, MC2R in the adrenal cortex, GR and MR in the hippocampus (GRh), the PVN and the pituitary. Unfortunately, many aspects of the axis have been ignored by the studies or have been observed in a non sex-specific manner, so that they couldn't be added to the table. Rodents data provided corresponds to experiments with non-handled pups or animal facility rearing pups as a control. Young is the period until weaning (≤ PND21 in rodents, ≤ 10-14 months in rhesus macaques, ≤ 18-21 years old in humans). Adulthood is generally observed from PND60 and on in rodents, 5 years old and on in primates and at about 25 years old and on in humans. The numbers correspond to bibliographic references.
In rhesus monkeys, Chen et al. [16] also proved that there might be an increase of basal CRH and GC secretion, in adult males peer-reared, following a definitive MS just after birth, but the increase in GC depends on the genotype.
In humans, the studies are consistent with these observations in animals, pinpointing that an early parental loss induces a higher cortisol awakening response (CAR) [34] and a higher response to the Dexamethasone/CRH test [35]. Although, in humans also, some studies even show that the CAR is reduced after an ELS [36]. However, the results in humans must be nuanced as the CAR is subjected to many criticisms (Box 1) and as the studies do not always separate the genders [35,36].
Consequently, early MS seems to induce a global basal and stress-induced hyperactivation of the HPA axis in adult men. In women, it might also be the case, but further studies should be done, taking into account the menstrual cycle.
How do those ELS-induced modifications of the HPA axis impact on aggression?
How is the HPA axis connected with aggression?
A big problem of studies concerning the HPA axis and aggression is that they poorly investigate on the causal relationship of those two aspects. They generally only give correlation factors. However, some say that the decision of aggressive behavior or non-aggressive behavior in front of a conspecific is determined by the testosterone/GC ratio [37]. A high ratio would make the balance tilt to aggression. This would in part explain the predominance of aggression in males in front of females. Some pinpoint also that a stress mediated by the PVN activation could inhibit the aggression in rodents [38]. In both cases, hyperactivating the HPA axis would thus reduce aggressiveness. Nevertheless, it looks more complicated as the injection of cortisol in humans seems to increase aggressiveness in women but not in men [39]. Thus, it underlies the necessity to study the modifications induced by an ELS specifically in each gender. Furthermore, basal level of GC seems important in relation with different types of arousal-related aggression (impulsivity for hyperarousal and GC deficient for hypoarousal) [40].
How each type of aggression might be affected by ELS and ELS-induced HPA axis modifications?
There is clearly a need of experiments in the field. Despite this, using the few studies which have been made and knowing how the HPA axis can be altered by an ELS might help us to understand how the different kinds of aggression can be affected by MS.
Females, in rodents, are generally aggressive only in the frame of maternal aggression. That means that only lactating females are concerned. Yet, we have seen that in female rats and mice, the HPA axis seems equally or more activated basally and after a stress after an early MS (Table 1). The studies which have focused on understanding maternal aggression suggest that CRH, AVP, ACTH, CRH as well as GCs inhibit maternal aggression [41–43]. Thus, MS, by increasing the stress-induced HPA axis tone, might reduce the emergency of maternal aggression. Nevertheless, further investigations are needed to verify whether the increased HPA axis tone due to MS is maintained during lactation. In rats, maternal aggression is indeed reduced after MS [1]. On the contrary, in mice, Veenema et al. [29] showed that adult females lactating after having been submitted to a MS had no increase of AVP in front of controls, but showed higher maternal aggression. The differences observed within rats and mice (Table 2) make it very difficult to draw any general conclusion on the consequences of MS on maternal aggression, as the mechanisms might be different. Furthermore, we know that in primates, on the contrary to rodents, females can display more kinds of aggression than the maternal one [15]. In addition to that, AVP, a component of the HPA axis, promotes maternal aggression when it is injected in the central amygdala, but not in the PVN [44]. The interactions of the HPA axis with the other areas of the brain have to be taken into account in future studies.
In humans, rhesus monkeys and rodents, MS seems to induce a higher HPA axis tone in males, both basally and after a stress (Table 1). Thus, we might expect less aggressiveness in males after early MS. In rodents, aggression is tested with the resident-intruder (RI) test. The resident-intruder (RI) test consists in putting a male in a cage with a female for quite a long time so as to create a sensation of residency. Then the female is retired before introducing a smaller intruder male. The resident generally attacks the intruder so as to protect his territory. This aggression is usually reported as an offensive aggression by the resident. In mice, Veenema et al. [29] showed indeed that the resident MS mice were less aggressive in the RI test than the non-MS controls. Surprisingly, male rats and rhesus monkeys appeared, on the contrary, whether more aggressive or equally aggressive after a MS (Table 2) in function of their genotype [15,16]. Thus, it seems that species and genetic particularities should also be taken into account for all kinds of aggression.
Table 2. Possible or effective consequences of early MS on HPA axis and adult aggression.
Species | Adult HPA axis stress-induced | Maternal aggression | Male offensive aggression | Defensive aggression |
Rats | +m/+f | -[1] | +? | |
Mice | +m/+~f | +[29] | -[29] | +? |
Rhesus | (nd)m/(nd)f | nd | +? | |
Humans | +m/~f | nd | nd | +? |
nd means not determined. + is for an increase of HPA axis activity for MS subjects in front of controls. ~ stands when there is no significant difference with control. - is for a decrease. ? is for the hypothesized changes given the data we have by now. m is for males and f for females. The numbers correspond to bibliographic references.
Defensive aggression is linked to an attack whether by a conspecific or by a predator. Thus, it is legitimate to think that it is linked to an activated HPA axis. Ebner et al. [45] showed that male intruders always show an increase in ACTH when set in the RI test. The most aggressive intruders are those who have a peak of AVP while the frightened ones are those with a decrease in AVP. Thus, it is tempting to hypothesize that defensive aggression may be linked to a specific activation of the HPA axis, especially to a raise in AVP. Thus, MS might stimulate defensive aggression.
Conclusion
The studies have shown that a MS generally induces a basal and a stress-induced hyperactivity of the HPA axis in males and eventually in females. Thus, this might reduce maternal aggression in rodents and intermale offensive aggression in rodents and primates. However, many components are involved in the aggression and looking only at the alterations of the HPA axis cannot fully explain the modulation of aggression after a MS. Much work remains to be done in the field: firstly by looking more scrupulously at the causal links between the HPA axis and aggression, secondly by studying the components of the HPA axis which were ignored by most studies (MC2R concentration in the adrenal cortex or CRHR in the pituitary gland, see table 1) and thirdly by leading more studies in primates.
Acknowledgments
I thank Dr Morgane Ollivier and Mrs. Audrey Denizot for their corrections and their advice concerning this review.
References and recommended reading
Papers of particular interest have been highlighted as:
● of special interest
●● of outstanding interest
1.
Veenema AH:
Early life stress, the
development of aggression and neuroendocrine and neurobiological correlates:
what can we learn from animal models?Front.
Neuroendocrinol. 2009,
30:497–518. [Full Paper][PubMed] This is the main review which focuses on the link between ELS and adult aggression with a
glance at the alterations of the HPA axis.
This is the main review which focuses on the link between ELS and adult aggression with a glance at the alterations of the HPA axis.
2. Markakis EA: Development of the neuroendocrine hypothalamus. Front. Neuroendocrinol. 2002, 23:257–291.
3. Keegan CE, Hammer GD: Recent insights into organogenesis of the adrenal cortex. Trends Endocrinol. Metab. 2002, 13:200–208.
4. Aisa B, Tordera R, Lasheras B, Del Río J, Ramírez MJ: Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology 2007, 32:256–266. [Full Paper]
5.
Nishi M, Horii-Hayashi N, Sasagawa T, Matsunaga W:
Effects of early life stress
on brain activity: Implications from maternal separation model in rodents
[Internet].
Gen. Comp.
Endocrinol. 2012,
doi:10.1016/j.ygcen.2012.09.024. [Full Paper] This review is of particular interest because it studies the different models of MS and
explains partly the differences observed in function of the protocol
used.
This review is of particular interest because it studies the different models of MS and explains partly the differences observed in function of the protocol used.
6. Champagne FA: Epigenetic mechanisms and the transgenerational effects of maternal care. Front. Neuroendocrinol. 2008, 29:386–397. [Full Paper]
7. Champagne FA, Curley JP: Epigenetic mechanisms mediating the long-term effects of maternal care on development. Neurosci. Biobehav. Rev. 2009, 33:593–600. [Full Paper]
8.
Lomanowska AM, Chatterjee-Chakraborty M, Steiner M, Kraemer GW:
Effects of motherless rearing
on basal and stress-induced corticosterone secretion in rat
pups. Stress
2011, 14:685–696. This article shows what might be the mechanisms by which the dam regulates the SHRP in
rodents.
9. Gunnar MR, Donzella B: Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology 2002, 27:199–220. [Full Paper]
10.
Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM:
Development of adult ethanol
preference and anxiety as a consequence of neonatal maternal separation in
Long Evans rats and reversal with antidepressant
treatment.
Psychopharmacology
(Berl.) 2001,
158:366–373. [Full Paper] There stands the protocol used in many following investigations to study the consequences
of MS.
11.
Zimmerberg B, Sageser KA: Comparison of two rodent models of maternal
separation on juvenile social behavior. Front.
Psychiatry 2011,
2:39. [Full Paper][PubMed] This article provides evidence that handling each pup in a different cage might induce
slightly different consequences on juvenile behavior of rats, than when the pups
are left all together or than control rats dam-reared.
12. Parr LA, Boudreau M, Hecht E, Winslow JT, Nemeroff CB, Sánchez MM: Early life stress affects cerebral glucose metabolism in adult rhesus monkeys (Macaca mulatta). Dev. Cogn. Neurosci. 2012, 2:181–193. [Full Paper]
13. Sánchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, Winslow JT: Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biol. Psychiatry 2005, 57:373–381. [Full Paper]
14. Karere GM, Kinnally EL, Sanchez JN, Famula TR, Lyons LA, Capitanio JP: What is an “Adverse” Environment? Interactions of Rearing Experiences and MAOA Genotype in Rhesus Monkeys. Biol. Psychiatry 2009, 65:770–777. [Full Paper]
15. Schwandt ML, Lindell SG, Sjöberg RL, Chisholm KL, Higley JD, Suomi SJ, Heilig M, Barr CS: Gene–Environment Interactions and Response to Social Intrusion in Male and Female Rhesus Macaques. Biol. Psychiatry 2010, 67:323–330. [Full Paper]
16. Chen G-L, Novak MA, Meyer JS, Kelly BJ, Vallender EJ, Miller GM: The effect of rearing experience and TPH2 genotype on HPA axis function and aggression in rhesus monkeys: A retrospective analysis. Horm. Behav. 2010, 57:184–191. [Full Paper]
17. Ventura-Junca R, Herrera LM: Epigenetic alterations related to early-life stressful events. Acta Neuropsychiatr. 2012, 24:255–265. [Full Paper]
18.
Weaver ICG: Epigenetic effects of glucocorticoids. Semin.
Fetal. Neonatal Med. 2009,
14:143–150. [Full Paper] In this review, Weaver describes very precisely the molecular mechanisms linking the
maternal tactile stimulation, the serotonin and the methylation of the GR
promoter in childhood.
19.
Aisa B, Tordera R, Lasheras B, Del Río J, Ramírez MJ:
Effects of maternal separation
on hypothalamic–pituitary–adrenal responses, cognition and vulnerability to
stress in adult female rats.
Neuroscience 2008,
154:1218–1226. [Full Paper] This is one of the few articles which focuses on the alterations of the HPA axis
specifically in females.
20. McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonté B, Szyf M, Turecki G, Meaney MJ: Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009, 12:342–348. [Full Paper]
21. Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM: Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 2008, 3:97–106.
22. Frodl T, O'Keane V: How does the brain deal with cumulative stress? A review with focus on developmental stress, HPA axis function and hippocampal structure in humans [Internet]. Neurobiol. Dis. 2012, doi:10.1016/j.nbd.2012.03.012. [Full Paper]
23. Fenoglio K, Brunson K, Baram T: Hippocampal neuroplasticity induced by early-life stress: Functional and molecular aspects. Front. Neuroendocrinol. 2006, 27:180–192. [Full Paper]
24.
Lajud N, Roque A, Cajero M, Gutiérrez-Ospina G, Torner L:
Periodic maternal separation
decreases hippocampal neurogenesis without affecting basal corticosterone
during the stress hyporesponsive period, but alters HPA axis and coping
behavior in adulthood.
Psychoneuroendocrinology
2012, 37:410–420. [Full Paper][PubMed] This article pinpoints the consequences of the MS on the hippocampal neurogenesis. It is
also of particular interest because it underlies the fact that MS induces not so
much modifications of basal HPA axis in childhood than modifications of the
adult HPA axis.
25. Daniels WMU, Fairbairn LR, Tilburg G, McEvoy CRE, Zigmond MJ, Russell VA, Stein DJ: Maternal separation alters nerve growth factor and corticosterone levels but not the DNA methylation status of the exon 17 glucocorticoid receptor promoter region. Metab. Brain Dis. 2009, 24:615–627. [Full Paper]
26. Macrì S, Würbel H: Developmental plasticity of HPA and fear responses in rats: A critical review of the maternal mediation hypothesis. Horm. Behav. 2006, 50:667–680. [Full Paper]
27.
Huot R: Foster
litters prevent hypothalamic-pituitary-adrenal axis sensitization mediated
by neonatal maternal separation. Psychoneuroendocrinology
2004, 29:279–289. [Full Paper] This article shows that a modification of the maternal behavior might be one of the major
facts explaining the consequences of MS in adulthood.
28. Desbonnet L, Garrett L, Daly E, McDermott KW, Dinan TG: Sexually dimorphic effects of maternal separation stress on corticotrophin-releasing factor and vasopressin systems in the adult rat brain. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2008, 26:259–268. [Full Paper][PubMed]
29.
Veenema AH, Bredewold R, Neumann ID: Opposite effects of maternal separation on intermale
and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin
and oxytocin immunoreactivity.
Psychoneuroendocrinology
2007, 32:437–450. [Full Paper][PubMed] This is one of the few articles which focuses on the link between MS, one aspect of the HPA
axis (AVP) and different kinds of adult aggression.
30. Pesonen A-K, Räikkönen K, Feldt K, Heinonen K, Osmond C, Phillips DIW, Barker DJP, Eriksson JG, Kajantie E: Childhood separation experience predicts HPA axis hormonal responses in late adulthood: A natural experiment of World War II. Psychoneuroendocrinology 2010, 35:758–767. [Full Paper]
31. Downs JL, Mattison JA, Ingram DK, Urbanski HF: Effect of age and caloric restriction on circadian adrenal steroid rhythms in rhesus macaques. Neurobiol. Aging 2008, 29:1412–1422. [Full Paper]
32. Fries E, Dettenborn L, Kirschbaum C: The cortisol awakening response (CAR): Facts and future directions. Int. J. Psychophysiol. 2009, 72:67–73. [Full Paper]
33.
Mourlon V, Naudon L, Giros B, Crumeyrolle-Arias M, Daugé V:
Early stress leads to effects
on estrous cycle and differential responses to stress. Physiol. Behav. 2011,
102:304–310. [Full Paper] This article shows that MS has consequences on the estrous cycle. It underlies the fact
that ELS consequences should be studied in a sex-specific manner and very
carefully in females in function of the phase of the estrous cycle.
34. Nicolson NA: Childhood parental loss and cortisol levels in adult men. Psychoneuroendocrinology 2004, 29:1012–1018. [Full Paper][PubMed]
35. Tyrka AR, Wier L, Price LH, Ross N, Anderson GM, Wilkinson CW, Carpenter LL: Childhood Parental Loss and Adult Hypothalamic-Pituitary-Adrenal Function. Biol. Psychiatry 2008, 63:1147–1154. [Full Paper]
36. Meinlschmidt G, Heim C: Decreased cortisol awakening response after early loss experience. Psychoneuroendocrinology 2005, 30:568–576. [Full Paper][PubMed]
37. Montoya ER, Terburg D, Bos PA, Honk J: Testosterone, cortisol, and serotonin as key regulators of social aggression: A review and theoretical perspective. Motiv. Emot. 2011, 36:65–73. [Full Paper]
38. Trainor BC, Sisk CL, Nelson RJ: 5 - Hormones and the Development and Expression of Aggressive Behavior [Internet]. In Hormones, Brain and Behavior (Second Edition). Edited by Editors-in-Chief: Donald W. Pfaff, Arthur P. Arnold, Susan E. Fahrbach, Anne M. Etgen and Robert T. RubinA2 - Editors-in-Chief: Donald W. Pfaff APA, Robert T. Rubin. Academic Press; 2009:167–205.
39. Böhnke R, Bertsch K, Kruk MR, Richter S, Naumann E: Exogenous cortisol enhances aggressive behavior in females, but not in males. Psychoneuroendocrinology 2010, 35:1034–1044. [Full Paper]
40. Haller J: The neurobiology of abnormal manifestations of aggression—A review of hypothalamic mechanisms in cats, rodents, and humans [Internet]. Brain Res. Bull. 2012, doi:10.1016/j.brainresbull.2012.10.003. [Full Paper]
41. Lonstein JS, Gammie SC: Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci. Biobehav. Rev. 2002, 26:869–888.
42. Nephew B, Bridges R: Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacol. Biochem. Behav. 2008, 91:77–83. [Full Paper]
43. Gammie SC, Seasholtz AF, Stevenson SA: Deletion of corticotropin-releasing factor binding protein selectively impairs maternal, but not intermale aggression. Neuroscience 2008, 157:502–512. [Full Paper][PubMed]
44. Albers HE: The regulation of social recognition, social communication and aggression: Vasopressin in the social behavior neural network. Horm. Behav. 2012, 61:283–292. [Full Paper]
45. Ebner K, Wotjak CT, Landgraf R, Engelmann M: Neuroendocrine and behavioral response to social confrontation: residents versus intruders, active versus passive coping styles. Horm. Behav. 2005, 47:14–21. [Full Paper]
46. Own LS, Patel PD: Maternal behavior and offspring resiliency to maternal separation in c57bl/6 mice [Internet]. Horm. Behav. 2012, doi:10.1016/j.yhbeh.2012.11.010. [Full Paper]
47. Conti G, Hansman C, Heckman JJ, Novak MFX, Ruggiero A, Suomi SJ: Primate evidence on the late health effects of early-life adversity. Proc. Natl. Acad. Sci. 2012, 109:8866–8871. [Full Paper]
48. Novak MA, Hamel AF, Kelly BJ, Dettmer AM, Meyer JS: Stress, the HPA axis, and nonhuman primate well-being: A review [Internet]. Appl. Anim. Behav. Sci. 2012, doi:10.1016/j.applanim.2012.10.012. [Full Paper]
49. Van Heerden JH, Russell V, Korff A, Stein DJ, Illing N: Evaluating the behavioural consequences of early maternal separation in adult C57BL/6 mice; the importance of time. Behav. Brain Res. 2010, 207:332–342. [Full Paper]
50. Zhang L, Hernández VS, Liu B, Medina MP, Nava-Kopp AT, Irles C, Morales M: Hypothalamic vasopressin system regulation by maternal separation: Its impact on anxiety in rats. Neuroscience 2012, 215:135–148. [Full Paper]
51. Lippmann M, Bress A, Nemeroff CB, Plotsky PM, Monteggia LM: Long-term behavioural and molecular alterations associated with maternal separation in rats. Eur. J. Neurosci. 2007, 25:3091–3098. [Full Paper]
52. Hamel AF, Meyer JS, Henchey E, Dettmer AM, Suomi SJ, Novak MA: Effects of shampoo and water washing on hair cortisol concentrations. Clin. Chim. Acta 2011, 412:382–385. [Full Paper]
53.
Wolfram M, Bellingrath S, Kudielka BM: The cortisol awakening response (CAR) across the
female menstrual cycle.
Psychoneuroendocrinology
2011, 36:905–912. [Full Paper] This article provides evidence that in humans also the HPA axis activity depends on the
phase of the menstrual cycle in women.
54. De Weerth C, Buitelaar JK: Cortisol awakening response in pregnant women. Psychoneuroendocrinology 2005, 30:902–907. [Full Paper]
55. Federenko I, Wüst S, Hellhammer DH, Dechoux R, Kumsta R, Kirschbaum C: Free cortisol awakening responses are influenced by awakening time. Psychoneuroendocrinology 2004, 29:174–184. [PubMed]
56. Griefahn B, Robens S: Cortisol awakening response - Are sampling delays of 15minutes acceptable?Int. J. Psychophysiol. 2011, 82:202–205. [Full Paper]
57. Veenema AH, Blume A, Niederle D, Buwalda B, Neumann ID: Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur. J. Neurosci. 2006, 24:1711–1720. [Full Paper][PubMed]
58. O'Mahony SM, Marchesi JR, Scully P, Codling C, Ceolho A-M, Quigley EMM, Cryan JF, Dinan TG: Early Life Stress Alters Behavior, Immunity, and Microbiota in Rats: Implications for Irritable Bowel Syndrome and Psychiatric Illnesses. Biol. Psychiatry 2009, 65:263–267. [Full Paper]


