Impact of large-scale environmental features changes on host-parasite interaction in marine and freshwater ecosystems.
2012/09/01
Abstract
Over the last half-century, changes in the weather have become the most complicated issue the world has to cope with. Global warming and environmental changes are not imminent but already manifest. Many ecosystems and ecological processes might be severely perturbed. Among these rising dangers is the prevalence of infectious diseases that is likely to become a major problem. An increase body of science has taken an active interest in the effects of global changes in host-parasite systems. This review shows the current knowledge of the impact of temperature and UVR exposure on this interaction, but also ocean acidification, salinity and species migration. A general trend cannot be drawn since this issue is highly case specific and the changes affect differently this system, but we underline the importance of considering multi-feature experiments in order to have a better understanding of the overall effect of global changes. Moreover, evolution processes may compensate the environmental effects and in another hand favor the strains that perform best under the new conditions.
Table of Contents
Introduction
According to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, a decadal 0.13°C increase has occurred since 1956 and is expected to reach 0.4°C within the next two decades [1]. Since climatic variables are driving many ecological networks, biological systems may be affected worldwide with respect to many ecological processes, changes in community structure and species interactions [2,3]. Increases in air and sea surface temperatures, as well as changes in the amount of solar radiation, sea level, precipitation, circulation patterns, ocean acidification and salinity are expected.
Among these consequences, many researchers have taken an active interest in potential effects of climate change and its different features on frequency and transmission dynamics of infectious diseases with rising temperatures. These changes are likely to become an important public health issue, especially for malaria and other vector-transmitted human diseases [4,5].
Since many climate change studies have focused on a single organism (i.e. shifts in phenology, physiology and changes in the distribution and range of species), analyzing biotic interactions such as host-parasite association is essential in order to attain a global understanding of the complexity of multilevel networks so that the potential impact of climate change on an entire ecosystem may be better understood.
Although parasites are commonly seen as threats to the viability of populations, they play a major ecological role and regulate host populations [6,7]. Since their life-cycle involve very often transmission from different invertebrate to vertebrate species via prey-predators networks, they form viable indicators of biodiversity, changes in ecosystem structure and function, environmental stressors, trophic interactions and climate conditions [8,9]. The extent and intensity of parasitism can be modulated by climatic or environmental conditions [10,11]. Therefore, changes in climate or in the local environment are bound to affect levels of parasitism with potentially important repercussions for host individuals, populations, communities and ecosystems, as demonstrated for instance by the predictive model of Kutz [12] for a parasitic nematode of muskoxen in a region especially sensitive to climate change: the Canadian Arctic. This model shows that temperature increase is likely to alter parasite transmission dynamics and thus increase infection pressure for muskoxen.
Throughout this review, we will look at different host-parasite models and favour the relevant model of a trematode parasite and its hosts as a generalized example as shown in Figure 1, and which involves two successive intermediate hosts and a final host in an intertidal system. Thus, different stages of the cycle will be considered separately because global warming and its indirect factors can affect each stage differentially.
- The output of cercarial transmission stages from snails
- Free-swimming cercarial survival and infectivity
- Susceptibility of second intermediate host (e.g. amphipod or cockle) to infections
- Host survival and parasite development within this host
Most parasites with complex life cycles have free-living stages, directly exposed to environmental conditions and thus are subject to the same changes as their host, but also indirect via effects on host sensitivity changes. Several larval stages could therefore be affected differentially by environment factors (see Figure 1) :
- Adult stages living in the intestine of mudflat affiliated birds
- Eggs passing out with the bird's feces and persisting in the environment until they may get ingested by a first intermediate host
- Free-swimming stages (cercarial) directly exposed to the environment, but not for long (< 24h, until they find a second intermediate host)
- Exposure in an indirect way through snail, mollusk which opacity might or might not let UVR reach the parasite, and bird hosts whose living conditions are affected by global warming
Pathogens are to cope with the same environmental conditions as their hosts but their responses and susceptibility might be different, causing likely destabilization of this system. An indicator of parasite transmission consists in measuring the survival and rate of transmission of free-living stages in order to estimate the basic reproduction ratio (Ro).
The intertidal ecosystem is a good model since parasites are ubiquitous components found in almost all animal species and are known to play crucial roles in the biodiversity and structure of this benthic community [11]. Also, intertidal organisms may be simultaneously exposed to a variety of physiologically stressful conditions. Since they already live in a physiologically stressful environment with extreme fluctuations that expose them to periods of emersion that can lead to severe desiccation, thermal stress and exposure to UV radiation, intertidal trematode parasites and their hosts are highly sensitive to environmental conditions and provide therefore an excellent tool for monitoring ecological impacts of climate change [11].
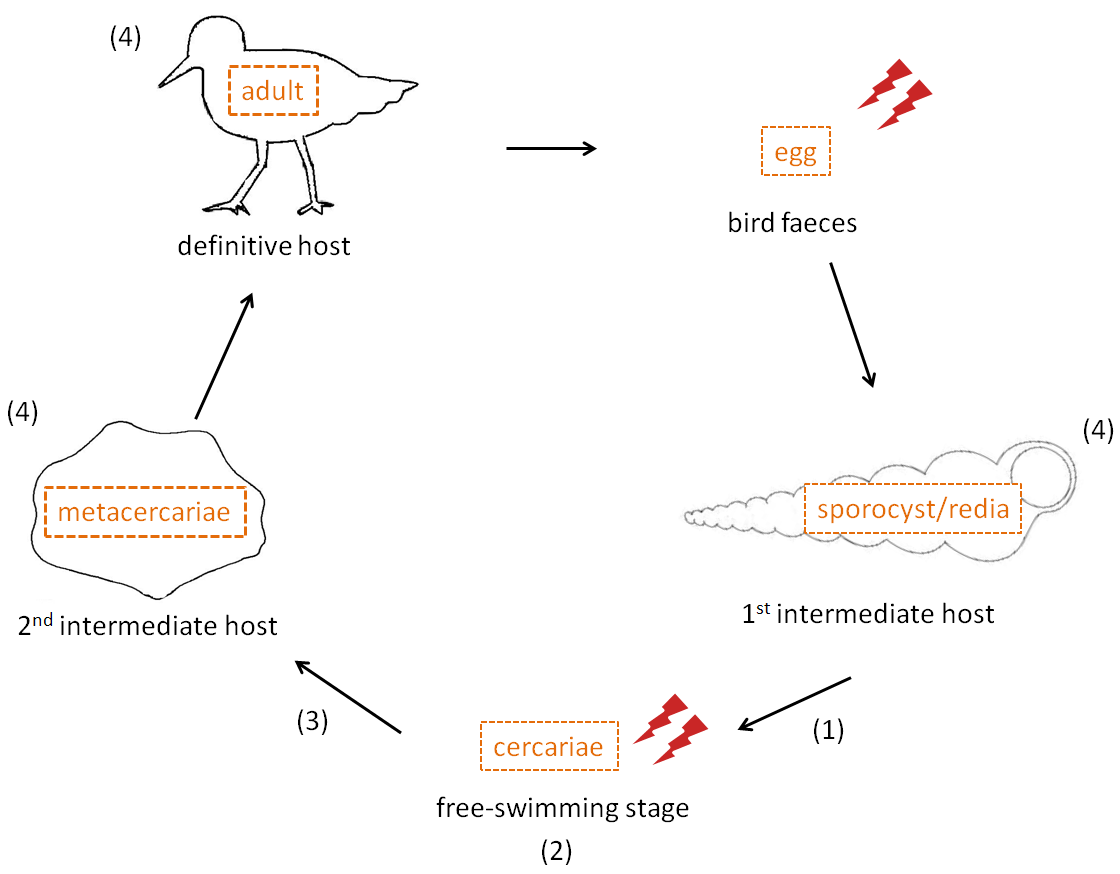
Figure 1. Life cycle and exposure to environmental conditions of different stages of intertidal trematode parasites (with the example of the trematode Curtuteria australis)..
Adults produce eggs inside shore birds which are expelled with bird faeces and then ingested by a first intermediate host (mudsnail or whelk). Asexual reproduction occurs within this host and produces a free swimming stage released during good environmental conditions, which then infects a second intermediate host (crustacean, cockle) that shore birds ingest. Stages that might be affected are (1) The output of cercarial transmission stages from snails (2) Free-swimming cercarial survival and infectivity (3) Susceptibility of second intermediate host (e.g. amphipod or cockle) to infections (4) Host survival and parasite development within this host. Lightning bolts represent stages directly exposed to environmental conditions such as UVR exposure or water salinity. Since parasites use ectothermic hosts, they are indirectly affected (e.g transmission rates) by environmental factors, especially temperature.
Temperature
Rising temperature is the most obvious factor of global warming. Several laboratory experiments have been undertaken in order to determine the effects of temperature on host-parasite systems.
Predictions ought to consider the sensitivity to temperature changes of parasites and their free-living stages, but of their hosts as well in order to anticipate the net effect of global warming on this system [13].
Different stages of parasites are temperature dependent (see Figure 2 for summary) and a model including the mortality of these stages (i.e. pre-infective stages, third stage larvae in the faeces and third stage on the pasture) reveals that larval stage and embryonation success are the most sensitive to temperature increase [14].
Commonly, cercariae production from the first intermediate host and infectivity of cercariae become more important with high temperatures with an optimum temperature [15,16] while their survival rate drops. Growth development is in fact significantly correlated with temperature [17]. However, these rates present an optimum as shown on Figure 2 meaning that increasing temperatures have a threshold effect on these parameters. These observations have led to the prediction that global warming might enhance the impact of trematodes through the increased number of infective stages present in a system [11,18].
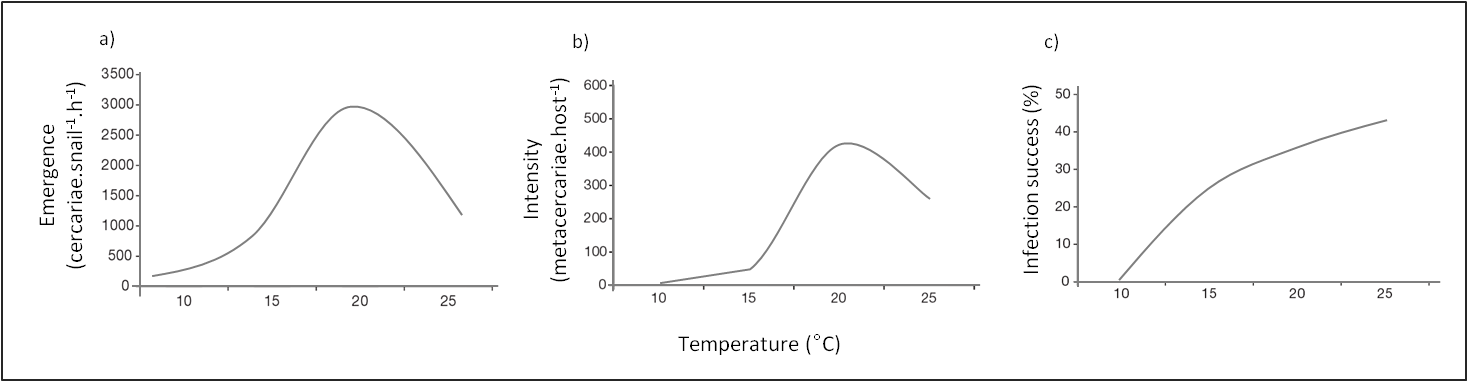
Figure 2. Temperature impacts on (a) emergence of cercariae from snail first intermediate host Littorina littorea, (b) infection intensity (metacercariae per host) of cercariae in Cerastoderma edule, (c) infection success percent of cercariae in cockle second intermediate host Cerastoderma edule. .
Moreover, with earlier timing of spring and warmer winter, parasites are exposed to longer growing seasons that would likely generate more generations annually and relax overwintering restrictions on pathogen life cycles. Transmission to definitive host is bound to take place even during colder months since birds visit the mudflat also in winter. Consequently, the number of generations may increase through two mechanisms : longer growing seasons and accelerated parasite development [5].
On the other side, we should consider hosts consequences as well. Temperature effects on hosts depend on the considered species, but usually as temperatures rise, hosts become more sensitive to infection, mainly explained by an immunosuppression and reduced virulence due to stress [19,20]. Thus, at the population level, it is bound to negatively affect the parasite's success at completing its life cycle.
A study on Maritrema novaezealandensis and its amphipod host Paracallio penovizealandiae shows that at low temperatures (< 20°C), transmission is low and amphipod survival unaffected. At intermediate temperatures (20 to 25°C), output and infectivity of cercarial transmission stages is at an optimum leading to an increase of parasite-induced mortality on amphipods that are affected directly by temperatures as well. At high temperature (> 30°C), transmission of the parasite is reduced (few cercariae, low infectivity, but temperature-induced mortality of amphipods is most pronounced) [21].
McCarthy [22] showed that decreased cercarial survival at high temperatures appears to be countered by improved infectivity likely mediated through increased cercarial activity resulting in a greater number of host contacts.
Overall, granted that temperatures do not exceed a certain optimum, moderately high temperatures are expected to benefit the transmission of the trematode including the parasite development within the mollusk hosts. This may, however, increase the risk of parasite-induced mortality of hosts. Moreover, if temperatures exceed an optimum level, direct negative effects on the parasite as well as the host may be pronounced.
However, a 20-year study on two farm fishes in Finland [23] has underlined the fact that the prevalence of infection is correlated with increases in temperature for some of the diseases (Ichthyophthirius multifiliis, Flavobacterium columnare), while in the other diseases, the pattern was the opposite (Ichthyobodo necator) or absent (Chilodonella spp.). Likewise, altered temperature experiments on the intertidal horn snail Cerithidea californica showed that the survival and activity of one trematode species remain unaffected with increased temperatures whereas another one decreased at the warmer temperature [24]. This opposite effect might be observed if the parasite optimal temperature for growth and transmission was lower [25]. In conclusion, increases in temperature may have differing effects on aquatic parasite-host dynamics depending on the biology of each disease and the role of local conditions.
UVR
Since the stratospheric ozone layer is reduced, more solar ultraviolet radiation (UVR) rays are likely to reach ecosystems, consequences of which are mostly increasing membranes, enzyme and DNA damages of exposed organisms ([26]. Even if the amount of UVR fluctuates with seasons, latitude, altitude, water depth, it has become more extensive with global warming [27]. This environmental stress factor could have an impact on aquatic organisms, as well on hosts as parasites through several mechanisms. Indeed, it has been shown that UV radiation can provokes an imbalance in the immune response to UV radiation, but it can also be benefit for the health (e.g. vitamin D production) but several studies of Ruelas et al. ( e.g. [28]) have underlined the fact that, even if parasite and infected snails are vulnerable to UVR exposure, both are able to repair UV-induced DNA damages. Biological and ecological effects of UVR have been well studied in aquatic ecosystems [29] and several UVR exposure studies has shown similar results; (i) host sensitivity to parasite is affected as their immune system is depressed and their resistance to infection is faulty [30] (ii) parasite survival decreases strongly because of free-living stages that are sensitive to environment conditions [30,31].
This effect is especially strong in clear, shallow waters. Consequently, intertidal ecosystems might be especially good UVR effects models as organisms are exposed to terrestrial atmospheric conditions during low tide. Indeed, different stages could be affected (see Figure 1): (1) eggs that are in bird's feces and exposed to the environment, (2) free-living cercariae stage, and (3) the second intermediate host, level of which depends on the transparency of the host (e.g. crustacean are less opaque than shellfish intermediate hosts).
However, a recent study on Daphnia dentifera (which is one of the more UVR-sensitive zooplankton group), and its virulent fungal parasite, Metschnikowia bicuspidate has revealed that although parasite infectivity is significantly negatively affected, Daphnia susceptibility to infection remains insensitive to UVR level. Therefore, solar ultraviolet radiation may reduce disease in this system. Indeed, if environmental stress kills parasites or reduces their infectivity without affecting host infection susceptibility, then disease should diminish as well [31].
Since hosts and parasites respond quite differently to UVR, it remains complicated to predict the overall effect of UVR on aquatic ecosystems. However, the fact that several zooplankton species are especially vulnerable to UVR is a potential risk for parasites that use them as intermediate hosts [32].
Ocean acidification and salinity
Acidification
With global warming, carbon dioxide is strongly released into the atmosphere by the ocean leading to a pH reduction of 0.28 to 0.7U [33] driving to a future pH of 7.4 – 7.8. Moreover, an acidification resulting of increasing in precipitation is expected [34]. The consequences of decreasing pH start to be understood and ocean acidification has been called as “global warming's evil twin” by Savitz [35] since it is a serious threat for calcifying marine organisms. Although it is well known that ocean acidification would affect marine organisms in terms of trophic interactions and biodiversity [36], few studies exist on this subject and the impact of changing pH on parasite-host interaction has never been studied.
The study of Koprivnikar [24] constitutes the first examination of the effects of pH on marine cercaria. It shows that the pH alone is not able to affect the cercaria but shows interaction with salinity and time. Indeed, the surviving and active E.californiensis cercaria in 35 versus 40 ppt only occurred in the higher pH (8.2).
Salinity
Few studies have focused on the effect of salinity on parasite although it is well known now that oceanic salinity will be altered with global warming mainly by decreased in ocean surface salinity [37] while rainfall and evaporation might either increase or decrease salinity.
In intertidal ecosystem, warm conditions and increased salinity are linked especially during low tide conditions when evaporation is high and temperature peaks : Sousa and Gleason [38] measured a salinity of 60 ppt (kg salt per kg water in parts per thousand) and temperature of 35°C during low tide in tide pools in Bolines Lagoon. Cercariae in this ecosystem might therefore have a high tolerance to a range of salinities, whereas marine ecosystems have a more stable level around 35 ppt.
Shift in salinity might have an impact on the distribution if host and parasite don't share the same tolerance range as reported with oysters and its sporozoan parasite Haplosporidium nelsoni [39]. Moreover, gradient salinity experiments show a significant effect on metabolism, ecdysis and physiology [40] as well as the reproduction since invertebrate larval stages are negatively affected by extreme salinity conditions (25 and 45 ppt) as a result of lipid peroxydation and oxidative stress [41].
Among the few parasite studies that has focused on the effect of salinity, it has been shown that cercarial longevity presents a high tolerance to varying salinity [42,43] and its emergence from mollusks can be influenced by salinity, the cercarial output becoming lower with low salinities [44,45]. The overall effect of higher salinity has been shown to be more beneficial to parasite than its host [46].
Host range, migration and species introduction consequences
Since parasites critically depend on successful transmission from one host to the next in their life cycle, death, unavailability or nest shift of a host before transmission will negatively impact the parasite as well. The seasonal dynamics of parasites and their hosts is therefore crucial if climate change affects host or parasite geographic ranges [47]. Biological invasion and shift in range is a significant consequence of global warming since various free-living animals (hosts) and their parasites have invaded recipient areas in which they had not previously occurred, threating the integrity and functioning of ecosystems [48,49]. Biological invasion can lead to emerging infectious diseases (EIDs) through the introduction and transmission of novel parasites from non-indigenous species to native species which are immunologically naïve [50–52]. Wildlife EIDs pose therefore a substantial threat to the conservation of global biodiversity. Poulin reviewed the impact of species introduction on the dynamic of endemic parasites [48] and several mechanisms can be listed: introduced species might (1) act as a competent hosts for endemic parasites leading to an increase in disease, (2) not be a suitable host for endemic parasites but be still infected leading to a sinks for parasite and a dilute disease risk for native hosts, (3) lead to a shift in one of the parasite's host abundance (e.g by competition or predation), (4) alter disease severity by changing the exposure or susceptibility of native hosts to infection by causing alterations in their behaviour or immunocompetence. Thus, climate change will not only caused species introduction but also local extinctions. A summary is illustrated on Figure 3.
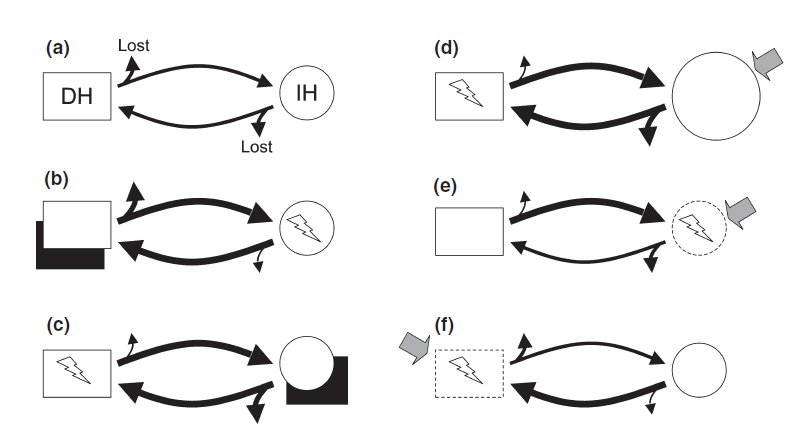
Figure 3. Summary of possible impacts of NIS on the dynamics of endemic parasitic diseases that might lead to disease emergence..
The hypothetical parasite considered here has a two-host life cycle involving a definitive host, DH (white rectangle), and an intermediate host, IH (white circle). During transmission from one host to the other, some parasites are unsuccessful and therefore lost from the system; the thickness of the arrows indicates the relative numbers that are either lost or successfully transmitted. The NIS (black rectangle) can either act as an alternative host for the parasite, or have an indirect effect (shaded arrow) on one of the native hosts. The native host incurring an emergent disease (i.e. an increase in infection rate) is indicated by a lightning bolt. (a) The situation prior to the invasion, providing a benchmark for comparisons. (b) The invader is a suitable alternative definitive host, more parasites there forereach a definitive host and more infective stages are produced to infect the intermediate host. (c) Same as the previous scenario except that the invader can serve as an alternative intermediate host, leading to greater infection risk for native definitive hosts. (d) The invader indirectly causes the population of native intermediate hosts to increase in size (larger circle), for instance by feeding on their predators or competitors, which leads to reduced losses at that stage of the parasite's life cycle, and greater infection risk for native definitive hosts. (e) The invader indirectly causes intermediate hosts to become more susceptible to infection, for instance by forcing them to change their microhabitat or diet, or via immunosuppression induced by stress. Induced habitat changes may be spatially extensive, such that the native species uses sub-optimal habitats in which infection risk is modified by other stressors. (f) Same as theprevious scenario except that the invader indirectly affects the definitive host instead of the intermediate host. (Adapted from Poulin et al. [48]).
Moreover, slight changes in distribution or abundance of any species belonging to the food web of any hosts will affect indirectly their parasites as well.
However, species migration and introduction is not the only hazard of host range shifts. In order to achieve the transmission and complete the life cycle of a parasite, a spatio-temporal overlap between host and parasite availability must occur. Indeed, several obstacles are introduced by global warming. First, temperature change can lead to a shift in synchronicity since seasonal distribution of marine species is often temperature dependent. Consequently, any change in seasonal condition such as earlier spring and warmer winter is likely to break the overlap between the host and parasite life cycle. A study on the digenean C. cooperi and mayflies, its second intermediate host, in Gull Lake by Esch et al. [53] is a relevant example showing that infection prevalence is affected by environmental conditions. Indeed, mayflies prefer deep waters but with the lake eutrophication, low waters became anoxic and mayflies distribution shifted to the high part of the lake where the first intermediate host stayed, leading to an increase in infection prevalence.
Moreover, Adam Ray et al. [54] suggests that a management strategy of a significant cause of juvenile salmonid mortality could be focus on disrupting the overlap of this parasite and its obligate hosts to improve emigration success and survival of juvenile salmon in the Klamath River.
Evolution and Adaptation
Coping with environment shifts, species are to adapt in order to survive and speciation events are likely to occur. Since temperatures will increase gradually over the next several decades, this will allow several parasite generations for natural selection to favor the strains that perform best under the new conditions. In this host-parasite system, coevolution is usually comparable to predator-prey system. A better adaptation of any organisms in the parasite cycle might lead to adaptation effect of the parasite, otherwise it is likely to disappear.
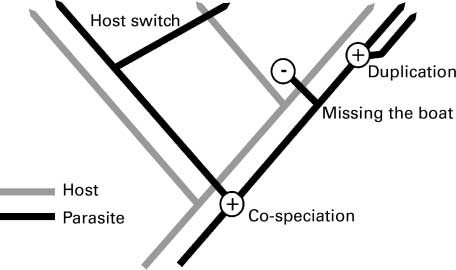
With shifts in geographical ranges and likely host or parasite extinctions, parasite might adapt to new conditions and new hosts. Selection pressure might lead to new species whereas other parasite might become extinct. Paterson [55] theorized the different evolutionary events generated by host migration and speciation (Figure 4). Thus, with geographical shifts, some parasites might “miss the boat” and become extinct and they are not able to adapt to another host. Otherwise, host switch would lead in some cases to parasite speciation.
Moreover, evolution may compensate the environmental change effects. Therefore, experiments, which involve placing parasites into completely new conditions and measuring the consequences, might be completely irrelevant because in future, when these conditions exist in nature, the parasites present will have gradually adapted to them.
Complexity of the study
In order to have a better understanding of the overall effect of climate change, it is necessary to have a detailed approach of the structure of ecosystems. Discrepancies between several studies on the effect of climate change on the extent of schistosome transmission [56,57] are due to the fact that this transmission is not only affected by the climate but also ecological, evolutionary, economic, social factors and most probably thinnest interactions with other organisms [58]. This example is relevant for all infectious diseases and vector-transmitted parasite studies that need to be approached with multifeatures considerations. Indeed, several authors have highlighted the need to better incorporate ecological complexity into climate change disease studies.
However, the impact of multiple stressors associated with climate change remains unclear and it is complicated to study this issue accurately because of the paucity of baseline data, the multivariate nature of climate change, and the nonlinear thresholds in both disease and climate processes. Associations between climate and disease do not necessarily imply causation, but results from correlative studies and short-term experiments can help us to separate the effects of climate from other components of global change.
Synergistic effects have been studied in order to have a more accurate approach (e.g: [24,59,60]). It is a minutely detailed work to quantify the magnitude of global warming different effects and it is impossible to predict the overall effect by manipulating isolated variables. For instance it has been demonstrated that the negative effects of UVR on some species could be exacerbated by ecologically realistic extreme salinities [61] and increased temperature [62]. Indeed, DNA repair mechanisms are more efficient at high temperature, which might reduce the negative effect of UVR.
Although studying synergistic effects is extremely complicated, it is an interesting way of predicting accurately the consequences of global warming on host-parasite system and is a crucial topic for further research.
Conclusion
Numerous changes provoked by global warming could affect free-living cercaria, survival, output, infectivity or transmission, but larval development and maturation as well. These changes could also have indirect effects on parasite via impact on host range and distribution, susceptibility to infection or population dynamic.
These different factors are affected differently and consequences of temperature increase and the other changes don't necessarily converge on the same trends (see Figure 5). Overall, warmer temperature is assumed to lead to an increase in cercarial output, enhancing the local impact of parasite unless its effects are cancelled out by other changes [18]. It is therefore necessary to conduct more studies on other features than temperature since it is not the only component of the current environmental changes, and as the review clearly shows, the different features are not equally studied. Moreover, it is necessary to conduct multivariate effects experiments in climate studies and evaluate the direct and synergistic effects of global environmental changes on this system in order to have a better understanding and predict accurately the impact of climate change on host-parasite interaction.
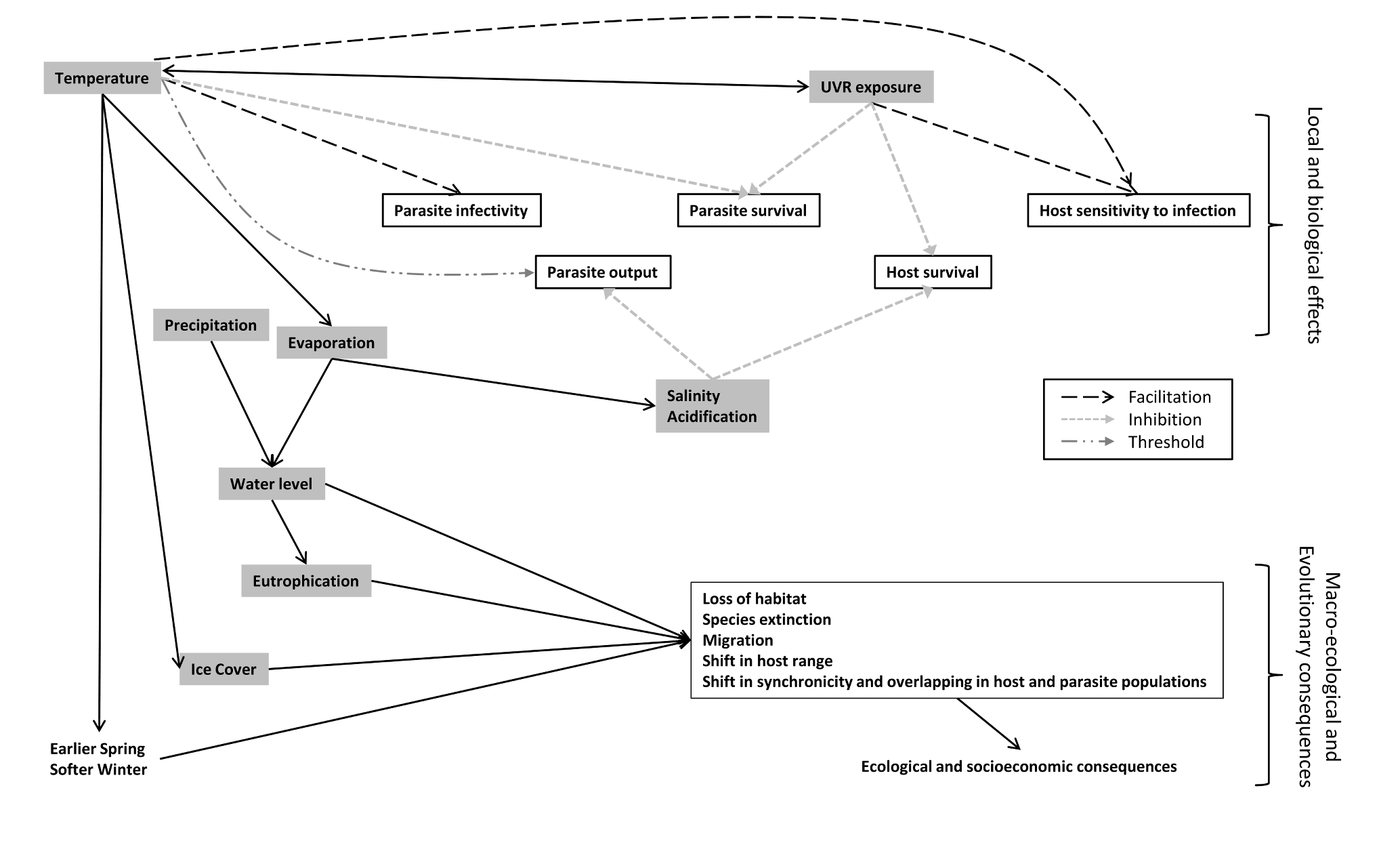
Figure 5. Interaction between global warming components and consequences on host and parasite interactions..
Small grey dashed lines indicate an inhibition effect whereas wider black lines indicate an enhancer effect. Threshold effect is represented by an alternation of short and large dotted line.
References and recommended reading
Papers of particular interest have been highlighted as:
● of special interest
●● of outstanding interest
1. IPCC: Intergovernmental Panel on Climate Change, 2007. IPCC Fourth Assessment Report (AR4)-Climate Change 2007: Synthesis Report. Geneva, Switzerland. 2007, [no volume].
2. Walther G-R, Post E, Convey P, Menzel A, Parmesan C,
Beebee TJC, Fromentin J-M, Hoegh-Guldberg O, Bairlein F: Ecological responses to recent climate
change. Nature 2002, 416:389–395. [Full
Paper]
3. Peñuelas J, Filella I, Comas P: Changed plant and animal life cycles from 1952 to 2000 in the Mediterranean region. Glob. Change Biol. 2002, 8:531–544. [Full Paper]
4. Lafferty KD: The ecology of
climate change and infectious diseases.
Ecology 2009, 90:888–900. [Full
Paper]
5. Harvell, Mitchell CE, Ward JR, Altizer S, Dobson AP,
Ostfeld RS, Samuel MD: Climate warming and disease
risks for terrestrial and marine biota.
Science 2002, 296:2158–2162. [Full Paper]
6. Hatcher MJ, Dick JTA, Dunn AM: How parasites affect interactions between competitors and predators. Ecol. Lett. 2006, 9:1253–1271. [Full Paper]
7. Larsen MH, Jensen KT, Mouritsen KN: Climate influences parasite-mediated competitive release. Parasitology 2011, 138:1436–1441. [Full Paper]
8. Marcogliese DJ: Parasites: Small players with crucial roles in the ecological theater. EcoHealth 2004, 1:151–164. [Full Paper]
9. Marcogliese DJ: Parasites of the
superorganism: Are they indicators of ecosystem health?Int. J. Parasitol. 2005, 35:705–716. [Full Paper]
10. Cattadori IM, Haydon DT, Hudson PJ: Parasites and climate synchronize red grouse populations. Nature 2005, 433:737–741. [Full Paper]
11. Poulin R, Mouritsen: Climate
change, parasitism and the structure of intertidal
ecosystems. J. Helminthol. 2006,
80:183–191.
12. Kutz S., Hoberg E., Polley L, Jenkins E.: Global warming is changing the dynamics of Arctic host–parasite systems. Proc. R. Soc. B Biol. Sci. 2005, 272:2571–2576. [Full Paper]
13. Marcogliese DJ: Implications of climate change for parasitism of animals in the aquatic environment. Can. J. Zool. 2001, 79:1331–1352.
14. Smith G: The population biology of the free-living phase of Haemonchus contortus. Parasitology 1990, 101:309–316. [Full Paper]
15. Mouritsen: The
Hydrobia ulvae–Maritrema Subdolum
association: Influence of temperature, salinity, light,
water-pressure and secondary host exudates on cercarial emergence
and longevity. J. Helminthol. 2002,
76:341–347. [Full
Paper]
16. Thieltges, Rick J: Effect of temperature on emergence, survival and infectivity of cercariae of the marine trematode Renicola roscovita (Digenea: Renicolidae). Dis. Aquat. Organ. 2006, 73:63–68.
17. Shiah FK, Ducklow HW: Multiscale variability in bacterioplankton abundance, production, and specific growth-rate in a temperate salt-marsh tidal creek. Limnol. Ocean. 1995, 40:55–66.
18. Poulin: Global warming and temperature-mediated increases in cercarial emergence in trematode parasites.Parasitology 2006, 132:143–151.
19. Harvell, Altizer S, Cattadori IM, Harrington L, Weil E: Climate change and wildlife diseases: When does the host matter the most?Ecology 2009, 90:912–920. [Full Paper]
20. Lafferty KD, Porter JW, Ford SE: Are diseases increasing in the ocean?Annu. Rev. Ecol. Evol. Syst. 2004, 35:31–54. [Full Paper]
21. Studer A: Parasites and global warming : net effects of temperature on an intertidal host-parasite system. 2011, [no volume].
22. McCarthy AM: The influence of temperature on the survival and infectivity of the cercariae of Echinoparyphium recurvatum (Digenea: Echinostomatidae). Parasitology 1999, 118 ( Pt 4):383–388.
23. Karvonen A, Rintamäki P, Jokela J, Valtonen ET: Increasing water temperature and disease risks in aquatic systems: Climate change increases the risk of some, but not all, diseases. Int. J. Parasitol. 2010, 40:1483–1488. [Full Paper]
24. Koprivnikar J, Lim D, Fu C, Brack SHM: Effects of temperature, salinity, and pH on the survival and activity of marine cercariae. Parasitol. Res. 2010, 106:1167–1177. [Full Paper]
25. Pounds JA, Bustamante MR, Coloma LA, Consuegra JA, Fogden MPL, Foster PN, La Marca E, Masters KL, Merino-Viteri A, Puschendorf R, et al.: Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 2006, 439:161–167. [Full Paper]
26. Tedetti M, Sempéré R: Penetration of ultraviolet radiation in the marine environment. A review. Photochem. Photobiol. 2006, 82:389–397. [Full Paper]
27. Häder D-P, Helbling EW, Williamson CE, Worrest RC: Effects of UV radiation on aquatic ecosystems and interactions with climate change. Photochem Photobiol Sci 2011, 10:242–260. [Full Paper]
28. Ruelas DS, Karentz D, Sullivan JT: Effects of UVB on interactions between Schistosoma mansoni and Biomphalaria glabrata. J. Invertebr. Pathol. 2009, 101:140–142. [Full Paper]
29. Bancroft BA, Baker NJ, Blaustein AR: Effects of UVB radiation on marine and freshwater organisms: a synthesis through meta‐analysis. Ecol. Lett. 2007, 10:332–345. [Full Paper]
30. Studer A: Effects of ultraviolet radiation on the transmission process of an intertidal trematode parasite. 2011, [no volume].
31. Overholt EP, Hall SR, Williamson CE, Meikle CK, Duffy MA, Cáceres CE: Solar radiation decreases parasitism in Daphnia. Ecol. Lett. 2012, 15:47–54. [Full Paper]
32. Rautio M, Tartarotti B: UV radiation and freshwater zooplankton: damage, protection and recovery. Freshw. Rev. J. Freshw. Biol. Assoc. 2010, 3:105–131. [Full Paper]
33. Caldeira K, Wickett ME: Oceanography: Anthropogenic carbon and ocean pH [Internet]. Nature 2003, 425. [Full Paper]
34. Schindler DW: The cumulative effects of climate warming and other human stresses on Canadian freshwaters in the new millennium. Can. J. Fish. Aquat. Sci. 2001, 58:18–29.
35. Savitz J, Harrould-Kolieb E: The oceans' acid test: can our reefs be saved? [Internet]. Front. Ecol. Environ. 2008, 6. [Full Paper]
36. Lannig G, Eilers S, Pörtner HO, Sokolova IM, Bock C: Impact of ocean acidification on energy metabolism of oyster, Crassostrea gigas—changes in metabolic pathways and thermal response. Mar. Drugs 2010, 8:2318–2339. [Full Paper]
37. NODC: National Oceanographic Data Center : Boyer TP, Antonov JI, Garcia HE, Johnson DR, Locarnini RA, Mishonov AV, Pitcher MT, Baranova OK, Smolyar IV (2006) World ocean database 2005. In: Levitus S (ed) NOAA Atlas NESDIS 60. US Government Printing Office, Washington. 2006, [no volume].
38. Sousa WP, Gleason M: Does parasitic infection compromise host survival under extreme environmental conditions? The case for Cerithidea californica (Gastropoda: Prosobranchia). Oecologia 1989, 80:456–464.
39. Crain CM, Silliman BR, Bertness SL, Bertness MD: Physical and biotic drivers of plant distribution across estuarine salinity gradients. Ecology 2004, 85:2539–2549. [Full Paper]
40. Shock BC, Foran CM, Stueckle TA: Effects of salinity stress on survival, metabolism, limb regeneration, and ecdysis in UCA pugnax. J. Crustac. Biol. 2009, 29:293–301. [Full Paper]
41. Deschaseaux ESM, Taylor AM, Maher WA, Davis AR: Cellular responses of encapsulated gastropod embryos to multiple stressors associated with climate change. J. Exp. Mar. Biol. Ecol. 2010, 383:130–136. [Full Paper]
42. Stunkard HW, Shaw CR: The effect of dilution of sea water on the activity and longevity of certain marine cercariae, with descriptions of two new species. Biol Bull 1931, 61:242–271. [Full Paper]
43. Mouritsen: The parasite-induced surfacing behaviour in the cockle Austrovenus stutchburyi: a test of an alternative hypothesis and identification of potential mechanisms. Parasitology 2002, 124:521–528. [Full Paper]
44. Koprivnikar J, Poulin R: Effects of temperature, salinity, and water level on the emergence of marine cercariae. Parasitol. Res. 2009, 105:957–965. [Full Paper]
45. Lei F, Poulin R: Effects of salinity on multiplication and transmission of an intertidal trematode parasite. Mar. Biol. 2011, 158:995–1003. [Full Paper]
46. Studer A, Poulin R: Effects of salinity on an intertidal host–parasite system: Is the parasite more sensitive than its host?J. Exp. Mar. Biol. Ecol. 2012, 412:110–116. [Full Paper]
47. Davis MB, Shaw RG: Range shifts and adaptive responses to quaternary climate change. Science 2001, 292:673–679. [Full Paper]
48. Poulin R, Paterson RA, Townsend CR, Tompkins DM, Kelly DW: Biological invasions and the dynamics of endemic diseases in freshwater ecosystems. Freshw. Biol. 2011, 56:676–688. [Full Paper]
49. Molnar JL, Gamboa RL, Revenga C, Spalding MD: Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ. 2008, 6:485–492. [Full Paper]
50. Taraschewski H: Hosts and
parasites as aliens. J. Helminthol.
2006, 80:99–128. [Full
Paper]
51. Daszak P, Cunningham AA, Hyatt AD: Emerging infectious diseases of wildlife - threats to biodiversity and human health. Science 2000, 287:443–449.
52. Dunn AM: Parasites and biological invasions.Adv. Parasitol. 2009, 68:161–184.
53. Esch GW: A long-term study on the population biology of Crepidostomum cooperi (Trematoda: Allocreadidae) in the burrowing mayfly, Hexagenia limbata (Ephemeroptera). 1986, [no volume].
54. Ray RA, Holt RA, Bartholomew JL: Relationship between temperature and C. Shasta-induced mortality in Klamath river salmonids [Internet]. J. Parasitol. 2011, doi:10.1645/para-/GE-2737.1. [Full Paper]
55. Paterson A: Host - Parasite Evolution: General Principles & Avian Models. Oxford University Press; 1997.
56. Yang GJ, Vounatsou P, Zhou XN, Tanner M, Utzinger J: A potential impact of climate change and water resource development on the transmission of Schistosoma japonicum in China. Parassitologia 2005, 47:127–134.
57. Martens WJ, Niessen LW, Rotmans J, Jetten TH, McMichael AJ: Climate change and vector-borne diseases: a global modelling perspective. Glob. Environ. Change 1995, 5. [Full Paper]
58. Yang G-J, Utzinger J, Lv S, Qian Y-J, Li S-Z, Wang Q, Bergquist R, Vounatsou P, Li W, Yang K, et al.: The Regional Network for Asian Schistosomiasis and Other Helminth Zoonoses (RNAS(+)) target diseases in face of climate change. Adv. Parasitol. 2010, 73:101–135. [Full Paper]
59. Przeslawski R, Davis AR, Benkendorff K: Synergistic effects associated with climate change and the development of rocky shore molluscs. Glob. Change Biol. 2005, 11:515–522. [Full Paper]
60. Studer A: Survival of an intertidal trematode cercaria : a multifactorial experiment with temperature, salinity and ultraviolet radiation. 2011, [no volume].
61. Karsten U, Dummermuth A, Hoyer K, Wiencke C: Interactive effects of ultraviolet radiation and salinity on the ecophysiology of two Arctic red algae from shallow waters. Polar Biol. 2003, 26:249–258.
62. Roos JC, Vincent WF: Temperature dependence of UV radiation effects on Antarctic cyanobacteria. J. Phycol. 1998, 34:118–125. [Full Paper]


