The post-reproductive lifespan: evolutionary perspectives.
01 - 01 - 2013
Abstract
Some metazoans, and humans first, present an interesting life history: they can survive after the cessation of there reproductive lifespan. This phenomenon is called menopause or more generally the post-reproductive lifespan (PRLS). The question is to know, how can this happen and why is it more beneficial to live longer when we do not reproduce anymore ? The factors influencing PRLS of metazoans is a well studied topic over the past decades. We reported a lot of factors increasing or decreasing PRLS among animals, mostly in mammals. There are non-evolutive explanations for the emergence of PRLS, which could be an artifact of the increasing lifespan. But two main evolutive theories have raised a lot of interests: [1] the grandmother hypothesis, which predicts that non reproductive mother help their daughter to reproduce, [2] the mother hypothesis, which assumes that mother stop reproducing to avoid risks related to childbirth, for her and their existing offspring. The discussion about the emergence of menopause still in debate.
Table of Contents
Introduction
Aging (or senescence) is a well studied topic for several decades. Hamilton (1966) [1], Williams (1957) [2] and Medawar (1946) [3], and Kirkwood (1979) [4] have addressed this issue and have builded the first theories about this phenomenon. Aging is defined as a «decline in performance and fitness with advancing age» [5]. Three theories have been proposed to explain why we die: the deleterious mutation accumulation theory [3,6], the antagonistic pleitropy theory [2] and the disposable soma theory [4,7,8]. The first two theories explain that deleterious mutations are accumulated because they have deleterious effects only in the late life, so that there is few selection on it. In the second theory, those mutations are selected because they have positive effects in early life. The third theory explains that there is a trade-off in the energy investments allocated for reproduction and for repairing the deleterious effects of the metabolism (oxidative catabolism), so the body age because we invest in reproduction.
It has been observed that the loss of reproductive ability can appear before death in metazoans. In some species, females stop to reproduce a short or a long time before they die. This phenomenon is called «menopause». Female menopause has received considerable attention over the past thirty years. Understanding the evolution of menopause is particular because of the common conception of the theory of evolution. According to the theory of evolution, surviving after the cessation of reproduction should not be selected, because it is completely useless regarding the fitness. Indeed, living species are « survival machines... for replicators (genes) » [9], which have to reproduce as much as possible to spread their genome, so we should reproduce and die when we couldn't reproduce anymore. Why non-reproductive individuals survive? Another formulation can be proposed: why selection didn't favor female who can reproduce longer? [1,2] Nowadays, studies have clearly shown that post-reproductive lifespan (PRLS) is common in animals, especially in mammals. But menopause seems to be only present in humans and some whales and most of studies published focuses on those species [10‑15]. Indeed, human females have much more to gain by stopping to reproduce and provide parental or grand parental care, than going on reproducing [10]. The prospects are huge, understanding the proximate causes and the evolutionary process of this dramatic physiological change could help us to manage with menopause, by finding biological models, elucidating the causes, or finding how comes that females live after their reproductive cessation.
It is important to distinguish the two terms «menopause» and «post-reproductive lifespan». Indeed, menopause is a biological term more related to humans, which is «the irreversible loss of the physiological capacity to produce offspring due to intrinsic biological factors» [16]. However we can extend this term to other mammals as cetaceans, which clearly show the same kind of reproductive cessation [17]. The biological definition of menopause includes the loss of fertility around a mean of 50 years in humans. The proximate cause of this loss is the cessation of ovulation accompanied by hormonal changes in neuroendocrine system [18] and the primary origin is the progressive depletion of ovarian follicles [19]. The term PRLS is a term used to describe «the time between age at reproductive cessation and age at death» (for individuals living beyond reproductive cessation) [16]. No study on PRLS focuses on the different strategies of « stopping to reproduce because of the cessation of ovulation » and « stopping to reproduce because it is just too costly ». To sum up, menopause is a biological term, which relies on a physiological state, while PRLS is just a time scale, between the time of reproductive cessation and the time at death [16]. So, menopause is included in PRLS. The two terms will be used during the discussion.
A lot of theories try to explain the emergence of menopause or PRLS (Table 1). There are non-adaptative theories and theories based on natural selection. Non-adaptative theories assume that PRLS could be just an artifact of life histories, such as the current raise of civilization for humans or the domestication for animals [2,20,21]. As a consequence, menopause should be just the deterioration of the reproductive system. An other explanation is that PRLS appeared as an insurance against indeterminacy [22]. On the other hand, there is two main theories, which assume that menopause is an evolutive process. The first is the Grandmother hypothesis, which proposes that non-reproductive mothers help their daughters to enhance their reproductive fitness. The second is the Mother hypothesis, which explains that old females stop reproducing because it is to risky for them, and so for their children.
Table 1. Summary of the main hypothesis currently proposed and their biological predictions.
|
Theory |
Biological predictions |
|
Non-adaptative theory |
PRLS is an artifact of increasing lifespan [2]. |
|
The «hazard hypothesis» |
PRLS in an insurance against indeterminacy: animal increase their total lifespan so much to benefit from all their reproductive lifespan that they outlive there reproductive lifespan [22]. |
|
The grandmother hypothesis |
Long living is selected because grandmother help their breeding daughters [23]. |
|
The «altriciality lifespan hypothesis» |
The increasing encephalization at childbirth has increased dependance of children, so helping behavior of grandmothers are selected [24]. |
|
The mother hypothesis |
Females, who stop to reproduce early are selected because they don't die at child birth and do not jeopardize the survival of existing offspring [25]. |
Here we discuss about the old and the recent theories of the evolution of PRLS and its evolutionary implications. First, we will discuss about who experiences menopause, which is still a debated topic. Second, we will successively analyze the evidence for and against the two main evolutive explanations of PRLS (the grandmother and the mother hypothesis), which have been the two main hypothesis supported for a long time. Then we will discuss about alternatives to the first two hypothesis and we will finally study the case of the emergence of menopause in humans.
Two aspects of PRLS and menopause
Who presents menopause or PRLS ?
It is generally admitted that only humans and sometimes cetaceans show menopause. This question is currently highly debated. Researchers investigated for instance if humans are the only primates who show menopause [26]. A few case of menopause have been found in wild and captivity: in rhesus macaques ( Macaca mulatta ) [27] and some other nonhuman primates exhibit menopause [20], as Japanese macaques [28].
In their paper, Walker and Herndon (2008) [26] concluded that several species experience menopause among nonhuman primates. Nonetheless, this conclusion seems to lie on the acceptation of the definition of menopause. They define «menopause in primates as the permanent, non-pathologic, age-associated cessation of ovulation» . But generally, it is difficult to know for sure that ovulation ceased, especially in animals. On the other hand, PRLS in nonhuman primates is very short compared to human's one, that is why ones could argue that other primates do not experience menopause [26]. This is actually a relevant topic, since nonhuman primates could be the closest model we have to study menopause [29]. The stress point of this discussion is that humans experience an extremely longer PRLS than every other primates. Indeed, 60% of the life of a human female can be accounted for PRLS, if we consider that the maximum lifespan is 122 years [26]. Studies have not shown that non-human primates experience menopause (here the cessation of ovulation a long time before death), but there is enough data to admit that they can live several years after their reproductive cessation (they can reproduce but do not), which means they have PRLS.
Numerous other species presenting PRLS have been found through the research on menopause. A few case have been found in insects, such as collembole [30], in birds [31], nematodes [32] or guppies [33], which are relevant models to study aging. As an example, the mean PRLS in guppies is 13,6% of the total lifespan and 16% in Japanese macaques [33,28]. Moreover, PRLS is particularly common among mammals and is present in many order (Ungulate, Primate, Carnivores, Cetacean, Rodent). Figure 1, reports PRLS for 35 different species of mammal. Many species here outlive their reproductive lifespan. This data show the importance of PRLS especially in mammals, but for a large part of species studied here, it still an assumption (explained by the maximum longevity recorded in the bibliography).
Figure 1. Lifespan of 35 populations of mammal.
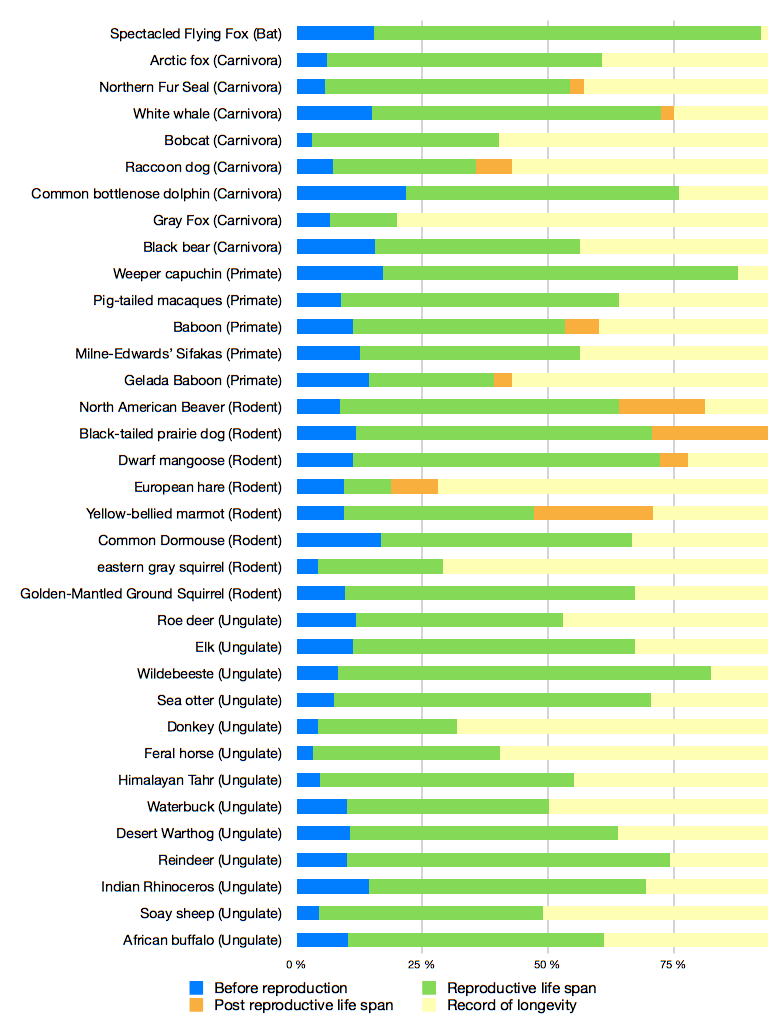
The different phases of the life of the populations are represented in percent: life time before reproduction (blue), reproductive time (green), and PRLS (orange and yellow), when animals do not reproduce anymore. We reported the maximum longevity for each species to complete data about the age of death. The record of longevity give us indications of the potential PRLS of species, knowing the cessation of the reproduction of the species studied. PRLS (in orange) give us the effective PRLS recorded.
The evolution of the mechanisms of menopause is not well known
The reproductive system in female mammals is a very complex system regulated by many hormones. Changes in the hypothalamic axis account for changes in reproductive physiology and the cessation of reproduction [34]. Williams (1957) [2] predicted that senescence was the result of pleiotropic effect, only punctual informations have been found for menopause. The p53 gene has been shown to be very important in reproduction and is not expressed at the same level and time in male and females mice [35] and p53 is a regulator o the basal expression of the LIF (leukemia inhibitory factor), which is an essential cytokine for successful blastocyst implantation: significant decreases in embryonic implantation, pregnancy rate and litter size are observed in p53−/− female. A decline of p53 gene is responsible for the cessation of reproduction.
The grandmother hypothesis
Nowadays, menopause is considered for a lot of researchers as a selective process. Mayer proposed in 1982 [36] one of the two principal theories, explaining the existence of PRLS and menopause. The grandmother hypothesis predicts that non-reproductive (or old) mothers can help their daughters to reproduce and breed children in order to enhance their own fitness. It means that surviving old mothers increase their inclusive fitness [36], for instance, because the presence of living grandmother has a positive effect on the number of grandchildren and on the fitness of those grandchildren [15]. Grandmothers enhance the survival of their grandchildren through protection and/or provisioning [23,37]. The more the grandmother is genetically close from his grandchild, the more she favors his survival, as measured Fox et al. (2010) [38]. So family with grandmothers are selected and PRLS is increased [11].
The case of humans is well studied and a good example to understand the evolution of PRLS and is particularly interesting because humans have a longer PRLS than other species, even other primates [26]. In humans it has been shown that, from an economic point of view (regarding the fitness benefits and the cost of the strategy used), this strategy of kin selection outweigh the cost of reproduction [10,15]. Cant and Johnstone (2008) [10] drew a model to explain the grandmother hypothesis and bound it to the conflict between generations in human. This model includes the cost of reproductive competition and the benefits of grandmothering. It predicts that menopause appears in old females because it becomes too costly to compete their own daughter to reproduce, and it is more beneficial for their inclusive fitness to help them to breed their children. As a consequence, the overlap of reproduction between generations in human is close to zero (Figure 2). This model comes as a complement to the grandmother hypothesis. Lahdenpera et al. (2004) [15] showed with a large study in Canadian and Finns population that human grandmothers actually increase their inclusive fitness by breeding their grandchildren. They studied the fitness benefits of post-reproductive women during the eighteenth and nineteenth centuries by measuring the effect of the presence of a grandmother on: number of grandchildren, reproductive performances of the offspring (lifetime fecundity, lifetime reproductive success) and grandchildren survival probability. Indeed, the presence of a grandmother has a positive effect on the number of grandchildren, as grandmother enhance the lifetime and the reproductive success of their offspring (earlier breeding, higher frequency, more success). All of these results show that selection can increase PRLS. Interestingly, the mortality rate of the grandmother increase dramatically when their daughter stop to reproduce. The role of demography and kinship has also been demonstrated in whales with the support of the grandmother hypothesis [39]. The social structure of the group increases the local relatedness between individuals (and particularly between females), which favors the evolution of menopause, because relatedness favors the probability of helping behaviors.
Figure 2. Overlap from three generation in four primates.
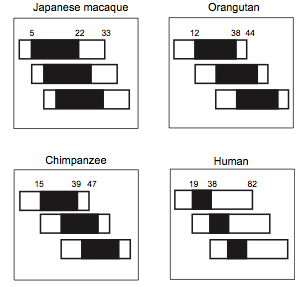
Rectangle account for the total lifespan and black parts account for the reproductive lifespan. (From Cant and Johnstone, 2008 [10]) .
In addition, Peccei (1995) [24] proposed the «altriciality lifespan» hypothesis, which is an alternative to the grandmother hypothesis. She identified encephalization and infant altriciality as the critical selection pressure, thanks to a demographic model measuring the extra-reproductive value on females. Infant born in more and more helpless states because of the increasing encephalization in Pliocene, so that helping behaviors are selected.
Most of the work in this field supports the grandmother hypothesis [23,37,40,10], although there is some criticism [41,13,20]. Adaptative hypothesis are balanced by non-adaptative hypothesis. Different authors found cues to contest the grandmother hypothesis. Indeed, some data support that the cessation of reproduction is not a selective process [41], but a by product of life histories, which increased dramatically in humans [20]. Packer (1998) [41] proved in two social species, baboon and lion, that the presence of the mother does not enhance the fitness of the offspring (survival, but also the number of children). Other authors argue that the inclusive fitness benefits are to small to influence natural selection [13], but they admit that phenomenons as allocare could influence life history.
Other cases of PRLS-like behaviors have been reported and can be considered as particular examples for the grandmother hypothesis: post-reproductive aphids become transformed into glue bombs (transformation of their abdomen, previously dedicated to embryo production, into a manufacture of defensives wax secretion), which can explode to protect their colony [30]. The point is that aphids live in clonal colony. So there are old helpers in the colony, which enhance the survival of their younger relatives.
The mother hypothesis
The hypothesis
The mother hypothesis is the second prevailing adaptative explanation of the existence of PRLS. This theory supports that post-reproductive life evolved to decrease the risk of dying by giving birth at old ages [42], it decrease also the risk of mortality for their existing children [23]. So females stop reproducing when this risk is to high. It is quite similar to the grandmother hypothesis: both are based on inclusive fitness provided through the care of children and the cessation of competition between generations. To sum up, Table 2 (taken in Pavard et al . 2008 [25]) resumes three main factors that can explain the evolution of menopause according to the mother hypothesis and how it comes to evolutionary changes.
Table 2. Variations in demographic parameters involved in the first level of the ‘‘mother'' hypothesis and their demographic and evolutionary outcomes. Some traits are not sufficient to explain menopause but have an impact, others are necessary to explain menopause. (Adapted from Pavard et al, 2008 [25]).
|
Variations in demographic parameters |
Demographic outcomes |
Evolutionary outcomes |
|
Increase in females' mortality with age |
Decreases the probability of having children at old ages |
Decreases the contribution of late reproduction to fitness (not sufficient) |
|
Increase in still-birth and the risks of birth defects with age |
Makes reproduction less efficient with age |
Decreases contribution of late reproduction to fitness (not sufficient) |
|
Increase in maternal mortality with age |
(1) Decreases the probability of having children at old ages (2) When linked with maternal care, decreases the survival of already born children because child survival until maturity is conditional on her mother's survival during the rearing time |
(1) Decreases contribution of late reproduction to fitness (not sufficient) (2) Increases the fitness benefit of a cessation of the reproduction strategy (necessary) |
Shanley and Kirkwood (2007) [23] showed in a gambian population that the strongest influence on child survival was the effect of maternal survival. This is significant during the first 2 years of life. This is just little evidence of the mother hypothesis, but it clearly fit with Table 1: the death of the mother decreases the fitness of the individual by making die their offspring. Logically, every trait which prevents mother to die when children are young, as menopause, will be selected. Figure 3, shows the increasing mortality risk at childbirth in Canadian and Finn populations and the predictions about mortality risk if the risks observed during child-bearing years are continued beyond menopause. The mortality risk is higher in young and old mothers.
Figure 3. Age-specific risk of dying from childbirth in preindustrial Finland (A, C) and Canada (B, D) during child-bearing years (16–50 years)
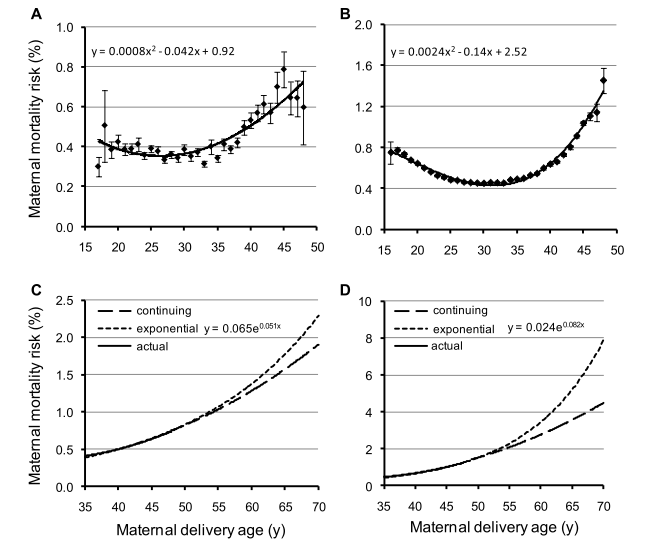
(A, B) and projected risks post-menopause (50–70 years) (C, D) The long-dotted lines (continuing function) show the projected risks of dying if the risks observed during child-bearing years are continued beyond menopause. The short-dotted lines show the risks of dying if the risk increases exponentially from the mean age at last reproduction (i.e., 38 years). (Reproduced from Lahdenperä et al, 2011 [40]) .
Testing the hypothesis
In humans, it is clear that availability of «natural data» can limit tests of these hypothesis. Moreover, many publications support that the mother hypothesis is not sufficient to explain the evolution of menopause: Rodgers (1993) [43], concluded that mortality during childbirth cannot account for menopause, thanks to models based on a 1906 Taiwanese population, because the rate of death during childbirth is too low to outweigh the benefits of a longer reproductive period. The mother hypothesis was recently tested by some authors with extensive tests, which investigates the consequences of the loss of the mother for the child. Lahdenperä et al . (2011) [40], find no support for this hypothesis in Canadian and Finns populations by measuring the child mortality risk with and without mother (but it is mostly supported by the fact that is those populations, the risk of mortality at child birth is very low). In addition, although mother is required to ensure offspring survival pre-weaning in humans, maternal loss there after can be compensated by other family members.
On the other hand, Shanley and Kirkwood (2007) [23], have used data on gambian families to test the effect of the grandmother and the mother on child. By the way, they have really interesting and valuable data because they used gambian village in the period 1950-1975, which could be representative of the population where first emerged menopause, as we will see in the study case in the end.
The environment influence PRLS
Insurance against indeterminacy
There are other theories, where there is no kind of inclusive fitness providing by helping behavior of the mother or the grandmother in those theories. It can be applied to species with no parental or no grandparental care. A new theoretical model has been proposed by Tully and Lambert (2011) [22], who describe the PRLS as an insurance against indeterminacy. Animals live longer in order to benefit from the all length of their reproductive lifespan, so that the somatic degenerescence comes after the reproductive degenerescence. Developmental variance (variance in reproductive and/or somatic lifespan and genetic variability) is a key factor, which increase PRLS in collembola ( Folsomia candida ), whereas the absence of developmental variance tends to decrease PRLS. Variance in the lifespan of somatic and reproductive cells has an additive effect on the length of PRLS.
Life histories influence PRLS
Regarding the different hypothesis proposed, the influence of many factors has been tested to understand what increase or decrease PRLS. Different aspects of the life of metazoans tend to predispose species to the evolution of menopause such as the group structures in whales [39], the mortality rate and a longer reproductive lifespan in guppies [33], the weaning age in humans [14]. In humans also it has been shown that life history strongly influence the reproductive lifespan of women [44]: selection favors an early reproduction where the mortality risk is high (in poor populations, where women suffer from high mortality when they become older). In general, the environment, the resources available and the mortality risk are influencing the strategy of reproduction in females. Females bet on the life time they invest in reproduction, to minimize the costs and maximize the fitness. Indeed, in case of higher mortality rate, mothers have to reproduce early to insure offspring. For guppies, a higher rate of predation leads to a longer reproductive lifespan: Reznik (2006) [33] found a correlation between the probability of an extended PRLS and the duration of the reproductive lifespan. To sum up, PRLS seems to be strongly influenced by the life history of each individual, which is full of hazard, influenced by different factors. With all this factors taken together, each population will invests a different part of his time in reproduction.
A study case. Humans: when did menopause appeared in humans ?
Is menopause a consequence of the increasing lifespan of humans, as a byproduct of the industrialization ? We may suppose that menopause appeared in humans because they lived longer thanks to modernization and medicine, what fit with the «artifact hypothesis», that is to say that menopause appeared because women lived longer and the reproductive system get exhausted [20,21,2]. But as some studies shown, menopause emerged when humans were already organized in hunter-gatherer populations [45]. They argue that the features of our species (large brain, long lives, parental investment in breeding, grandparental support, long child dependency of parents) appeared before the preagricultural history [46]. This a reason why today, scientists test their hypothesis on the evolution of menopause on current hunter-gatherer populations [47] or populations living in developing countries [48]. There is some evidence that menopause in humans did not appeared because of civilization and modern circumstances, or the effect of agricultural and industrial economies [48]: an emergence because of civilization could be too near from today to be the result of natural selection, as he says. So there is a lot of speculations on the date of the apparition of human menopause. Hawkes (1998) recorded three dates for life history changes mediated by increasing PRLS: the initial appearance of Homo erectus 1,8 millions years ago, in early Homo sapiens 600,000 years ago, or 50,000 years ago, which is when the Homo sapiens had a huge ecological and competitive success compared to the other Homo .
Conclusion and further research
Here is still a debate about how appeared menopause, and more generally PRLS. If only humans and whales clearly show menopause, PRLS is not unusual in mammals. Many theories converge to conclude that PRLS evolved because it is adaptative and because it increases the fitness of animals who dispose of this feature. So non-reproductive mother increase their inclusive fitness, through the protection and the help of their offspring. On the other hand, there is also evidence for non-adaptative theories: the increase in life span gave the possibility to use all the ovocyte stock.
Other factors influencing PRLS have been shed in light in the past ten years (mortality risk for example), which can explain the variations of PRLS in populations, in animals as well as in humans. Indeed the duration of PRLS can change from a population to another in a species. Humans, for example, is a species, which can be divided in many different populations, in which life histories vary, because of different life styles, environment, or wealth.
The fact that PRLS in particularly commons in mammals should be more investigated. Indeed, mammals have a lots of different life-histories: longevity, size, body mass, weaning age, social structure vary between species. We can assume that PRLS could be bound to the social structure in mammals. Indeed, if we agree with selective based theories as the grandmother and the mother hypothesis, the emergence of this feature based on parental care, helping behavior require a minimal social context. Animals had to live together to provide care to weaned, adult or sub-adult offspring. So it could be very interesting to investigate if the sociality level could influence PRLS. One prediction that could be made is that PRLS should be more selected in highly social species. Indeed, the more social is a species, the more high is the probability to find helper in the group, which can enhance the new-born and young survival. Moreover, the helper may be or should be, if we refer to the theory of the «selfish gene» [9], related to the offspring helped. So, non-reproductive grandmothers should be more selected more in social species.
Finally, there is some evidence for each theory and also against (summarized in Figure 4). May be should we consider that more than one theory could account for the emergence of menopause. We can imagine a mixed scenario between non-adaptative and adaptative hypothesis: an increase in female lifespan should have lead to a longer presence of grandmother, and increased the fitness and the lifespan of those family thanks to helping behaviors of the grandmother, leading finally to the emergence and the increase of PRLS. Moreover, a favorable environment or less predators could have allowed humans and whales to increase their lifespan and PRLS appeared. Indeed, we can have such phenomenon in captive animals as bird, in which we observed PRLS [31]. According those assumptions, PRLS could have appeared several times independently.
Figure 4. Summary of the different theories on PRLS.
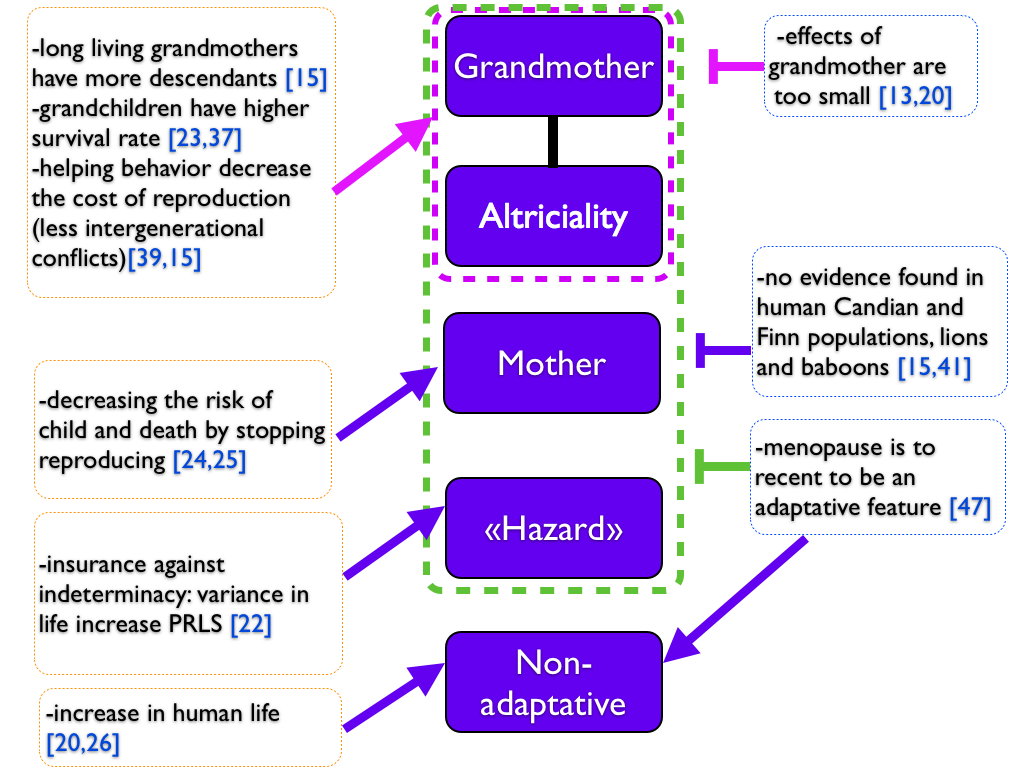
Five reported hypothesis are reported in the middle, while evidence for and against are on the right and on the left. Arrows go from evidence to an hypothesis. Vertical bars account for evidence against an hypothesis. Dotted lines group theories together: adaptative hypothesis in green, hypothesis related to the grandmother hypothesis in purple. Colors helps to indicate which hypothesis or which group of hypothesis arrows and vertical bars point.
Aknowledgements
I want to thank Aurélie Cohas and Jean-François Lemaitre for helping me to write this report and also Ben, Marion and Vérane, who helped me to deal with LateX and made me suggestions.
References and recommended reading
Papers of particular interest have been highlighted as:
● of special interest
●● of outstanding interest
2.Williams GC. Pleiotropy, Natural Selection, and the Evolution of Senescence. Evolution 1957, 11 :398-411.
4.Kirkwood TBK, Holliday R. The evolution of ageing and longevity . Proceedings of the Royal Society B 1979, 205 :531-546.
5.Hughes KA, Reynolds RM. Evolutionary and mechanistic theories of aging. Annual review of entomology 2005, 50 :421-445.
7.Kirkwood TBL, P. Kapahi, Shanley DP. Evolution, stress, and longevity. Journal of anatomy 2000, 197 Pt 4 :587-590.
10.Cant MA, Rufus AJ.
Reproductive conflict and the separation of reproductive generations in
humans.
Proc. Natl .Acad. Sci. USA
2008,
105
:5332-5336.
●●
11.Hawkes K, O'Connell JF, Jones NG, Alvarez H,
Charnov EL.
Grandmothering, menopause, and the
evolution of human life histories.
Proc. Natl .Acad. Sci. USA
1998,
95
:1336-1339.
●●
12.Herndon JG. The Grandmother Effect : Implications for Studies on Aging and Cognition. Gerontology 2010, 56 :73-79.
13.Kachel F, Premo LS, Hublin JJ.
Grandmothering and natural selection.
Proceedings. Biological sciences / The Royal
Society
2010,
278
:384-391.
●●
14.Kachel F, Premo LS, Hublin JJ. Modeling the effects of weaning age on length off male reproductive period: implications for the evolution of human life history. American journal of human biology : the official journal of the Human Biology Council 2011, 23 :479-87.
15.Lahdenpera M, Lummaa V, Helle S, Tremblay M, Russell
AF.
Fitness benefits of prolonged post-reproductive
lifespan in women.
Nature
2004,
428
:178-181.
●●
16.Cohen A. Female post-reproductive lifespan : a general mammalian trait. Biol. Rev. 2004, 79 :733-750.
17.Johnstone RA, Cant MA.
The evolution of menopause in cetaceans and humans: the
role of demography.
Proceedings. Biological sciences / The Royal
Society
2010,
277
:3765-3771.
●●
19.Vermeulen A. Environment, human reproduction, menopause, and andropause. Environmental health perspectives 1993, 101 :91-100.
20.Pavelka MSM, Fedigan L. Menopause: A Comparative Life History Perspective. Yearbook of Physical Anthropology 1991, 38 :13-38.
22.Tully T, and Lambert A.
The evolution of postreproductive lifespan as an insurance
against indeterminacy.
Evolution
, 2011,
65
:3013-3020.
●
23.Shanley DP, Sear R, Mace R, Kirkwood TBL. Testing evolutionary theories of menopause. Proceedings of the Royal Society B 2007, 274 :2943-2949.
24.Peccei J.
The origin
and evolution of menopause: The altriciality-lifespan hypothesis.
Ethology and Sociobiology
1995,
16
:425-449.
●
25.Pavard S, Metcalf EE. Senescence of reproduction may explain adaptive menopause in humans: a test of the "mother" hypothesis. American journal of physical anthropology 2008, 136 :194-203.
26.Walker ML, Herndon JG.
Menopause in nonhuman primates?
Biology of reproduction
2008,
79
:398-406.
●
27.Johnson RL, Kapsalis E. Menopause in Free-Ranging Rhesus Macaques : Estimated Incidence , Relation to Body Condition , and Adaptive Significance. International Journal 1998, 19 :751-765.
28.Takahata Y.
Do the
Old Aged Females Experience A Long Post-reproductive Life Span ?: The Cases of
Japanese Macaques and Chimpanzees.
Primates
1995,
36
:169-180.
●
29.Bellino FL. Nonhuman primate models of menopause workshop. Biology of Reproduction 2002, 68 :10-18.
30.Foster WA. Behavioural ecology: the menopausal aphid glue-bomb. Current biology 2010, 20 :R559-560.
31.Holmes DJ, Ottinger M. Birds as long-lived animal models for the study of aging. Experimental Gerontology 2003, 38 :1365-1375.
32.Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech. Ageing Dev. 1977, 6 :413-429.
33.Reznick D, Bryant M, Holmes D. The Evolution of Senescence and Post-Reproductive Lifespan in Guppies (Poecilia reticulata). PLoS Biology 2006, 4 :e7.
34.Rashidi A, Shanley D. Evolution of the menopause: life histories and mechanisms. Menopause international 2009, 15 :26-30.
35.Hu W, Feng Z, Teresky AK, Arnold JL. p53 regulates maternal reproduction through LIF. Nature 2007, 450 :721-724.
37.Foote AD. Mortality rate acceleration and post-reproductive lifespan in matrilineal whale species. Biology letters 2008, 4 :189-191.
38.Fox M, Sear R, Beise J, Voland E, and Knapp. Grandma plays favourites: X-chromosome relatedness and sex-specific childhood mortality. Proceedings. Biological sciences / The Royal Society 2010, 2010:567-573.
39.Johnstone RA, Cant MA.
The evolution of menopause in cetaceans and humans : the
role of demography.
Proceedings of the Royal Society B
2010, :3765-3771.
●●
40.Lahdenperä M, Russell AF, Tremblay M, and Lummaa V.
Selection on menopause in two premodern human
populations: no evidence for the Mother Hypothesis.
Evolution; international journal of organic
evolution
2011,
65
:476-489.
●
41.Packer C, Tatar M, Collins A. Reproductive cessation in female mammals. Nature 1998, 392 :807-811.
42.Grimes D. The morbidity and mortality of pregnancy – still risky business. Am J Obstet Gynecol 1994, 170 :1489-1494.
44.Pettay JE, Helle S, Jokela J, Lummaa V. Natural Selection on Female Life-History Traits in Relation to Socio-Economic Class in Pre-Industrial Human Populations. Plos one 2007, 7 :e606.
45.Gurven M, Kaplan H. Longevity among hunter–gatherers: a cross-cultural examination. Population and Development Review 2007, 33 :321-365.
46.Kaplan AJ, Robson HS. The emergence of humans: the coevolution of intelligence and longevity with intergenerational transfers. Proc. Natl .Acad. Sci. USA 2000, 99 :10221-10226.



