Propositions for a phylogenetic approach to social learning
01 - 11 - 2012
Abstract
Social learning is an ability shared by many animals, recently widened to unexpected taxa such as Drosophila ; and may have important consequences on evolutionary processes. Surprisingly, whereas learning and social learning are studied by a wide range of disciplines, little has been done on the phylogeny of these abilities. Recently, several authors proposed methods for using phylogeny in cognitive sciences. We argue that such an approach could bring a lot to the understanding of cognitive processes underlying social learning. In particular, establishing evolutive history of characters could help with the lively debate of general process and special adaption.
Table of Contents
Introduction
That animals can learn is known for ages but this field of research is today very active, involving various disciplines (Figure 1). For over a decade, increasing efforts have been made to integrate all those approaches together [1–3] (see Figure 1). Interestingly, research on animal learning and social learning, establish strong bridges with human sciences.
Different definitions of learning can be found in the literature and according to Shettleworth [1] none can be entirely satisfactory. A general broad definition like acquisition and maintenance of new information will probably be sufficient for us. This definition is modified after Dukas's definition which insists on neuronal representations of new information [3]. Social learning would be the learning from other individuals. But as discussed in Fragaszy-Perry [4], finding an exact definition to social learning can also be very problematic. One solution is to consider that social learning is learning affected by a social relation, or to say it differently, that would not have been equal without a social relation. It is opposed to individual learning. Such a broad definition covers in fact very different psychological mechanisms from simple stimulus enhancement (see below) to imitation [5].
As pointed out by several authors [1,3,4] studies of cognitive processes (among which learning is a crucial part) can only be led by observing their behavioral output. Studying cognition implies studying behavior. In 1963 Tinbergen [6] proposed a still useful scaffold to the study of behavior. A behavior can only be comprehensively understood through answering to 4 major questions (Figure 1): what are the mechanisms underlying this behavior (proximate mechanisms: cognition; and distal mechanisms: neuroscience, genetics…)? What is the influence of ontogeny (development, plasticity…) on it? What is (are) its function(s) ( adaptative values , primarily the domain of behavioral ecology)? What are the phylogenetic constraints-determinisms? This 4-axis approach is still useful for cognitive-centered approaches (Figure 1) as for all phenotypic traits in general [6].
Figure 1. Schematic of the Tinbergen's four questions applied to the biological study of behavior.
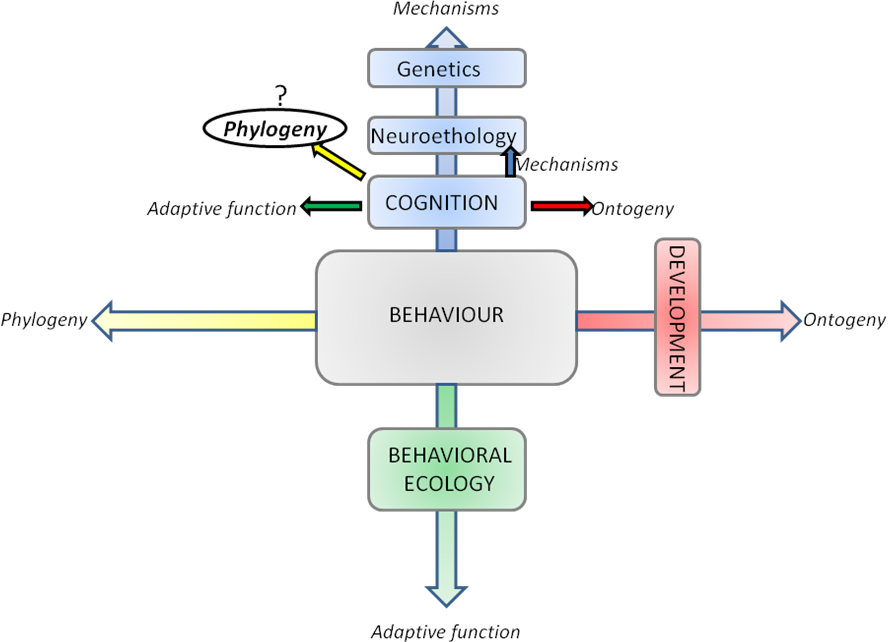
In italics are the 4 main axis that one should investigate together to have a comprehensive understanding of a behavior. Tinbergen considered this methodological scaffold relevant for the study of all phenotypic traits and it could in particular be applied to cognitive-centered studies. Some particular axis corresponds to active specialized disciplines (some appear in colored squares). For some years there have been increasing efforts to integrate those disciplines together, and it should be emphasized that this four axis distinction is by no mean a strict delimitation, but rather a tool to remember all biological aspects of importance. The object of this report is to examine how phylogeny is used for the study of a cognitive capacity: social learning. (Adapted from [6]) .
In particular, some behavioral characters can be employed for building phylogenies or merely for retracing their evolutive history (see [7]). Still, it appears that phylogeny is less employed in behavioral studies than for morphological or molecular characters [8]. It seems that it is even less employed in cognitive research, but very recent articles tackle this issue [9,10]. Fitch et al. [9] in particular wondered about the origin of social learning.
Biological significance of social learning
Many specific cognitive mechanisms have been proposed in the literature to describe how social learning can occur (see [5] for a review). For instance in imitation, an individual reproduces the sequences of an action after observing another performing it (also applied to vocal learning, see Box 1 for the example of vocal imitation in birds) and in local enhancement, an individual is attracted to a place by the presence of another and is thus exposed to the same specific local stimuli. Imitation could be underlined by specific neuronal schemes, whereas local enhancement could be individual learning triggered by a social interaction. It could be interesting to investigate whether we can find a phylogenetic base for all these different proposed mechanisms, and their relation to individual learning mechanisms.
Social learning can sometimes lead to traditions even in non-human animals. The definition of tradition (or depending on authors of culture) is highly debated but the one given by Fragaszy and Perry [4] is often cited: “a distinctive behavior pattern shared by two or more individuals in a social unit, which persists over time and that new practitioners acquire in part through socially aided learning.”
Many theoretical works have been led on the evolutive implications of social learning and traditions, for instance to determine when, in theory, it is profitable to use social instead of individual learning (both have their advantages and costs; [11,12]). Moreover the possibility of a cultural transmission has also been modelled [13,14]. The concept of traditions implies a stable transmission of information among individuals, possibly across generations. It could therefore be seen as an inheritance system, parallel to the genetic inheritance, along with other processes like epigenetic inheritance (see [14] for a review). Cultural transmission is a major force in the human lineage [15], but could also be important in non human animals. For instance vocal dialects in Birds have long been thought to be an actor of speciation. In Cetaceans, it has been observed that population genetic structures of Sperm Whales reflect more vocal dialects than geographical repartition [16]. An important question is whether all processes of social learning [5] can contribute equally to traditions. Knowing the phylogenetic distribution and appearance of those different mechanisms could be of importance, and it appears that social learning and traditions are more widespread than previously thought.
Social learning abilities within the phylogenetic tree of life: current knowledge
So far social learning has been reported in Vertebrates (Teleosts, Turtles, Birds, Mammals) and in Ecdysozoans (Insects [17,18]). Despite the fact that major biological models for individual learning (ie C.elegans , Aplysia ) exists in Lophotrochozoans, octopi are as far the only representative of that major branch for which social learning has been suggested [19] to our knowledge. However, the other major biological model, Drosophila melanogaster , shows interesting results: Mate choice copying has been reported in female fruit flies [17]. It has been suggested that mate choice copying (like vocal learning) could be a particularly relevant context for cultural transmission to impact evolution, because of stable specific sexual or natural selection pressure it creates [12]. Females Drosophila also learn oviposition site preferences [18] from conspecifics. Experiments suggested that this transmission was performed by direct social interactions between flies rather than stimulus or local enhancement, and that preferences were maintained in second-order observers (observing the first observers) thus could be a form of tradition [18].
It is generally believed that all animals with nervous cells should have a form of learning ability [3]. Whether learning can occur in other clades is poorly documented, and would strongly depend on the definition of learning adopted [1]. Considering the hypothesis that many processes are shared between social and individual learning; social learning could also occur in most nervous animals.
Social learning and sociality
Another hypothesis is that social learning is a specific feature developed by social animals (see [20]). Wilkinson et al. [21] challenged this view arguing that a non-social turtle ( Geochelone carbonaria ) appears able to learn from conspecifics. Even though those turtles were grouped some months before the experience (to “socialize” them), in their natural behavior they are not considered social animals.
However, the authors do not discuss the sociality of related turtle species nor their social learning abilities (which are probably unknown). Maybe the ancestor was a social species that could learn from conspecifics, and whose ability has been inherited. One could argue that cognitive abilities are labile and special adaptations thus would have disappeared when turtles became solitary. But in this case, how much labile is it? The classical approach would be to search for a correlation between social learning and sociality among much more taxa, but few studies of social learning in solitary species have been led [9,21]. In this approach the evolutive histories of both traits are not needed to be known but phylogenetic correction has to be considered.
Phylogenetic signal and comparative approaches
In the so called evolutionary approach, or special adaptation approach, which focus on the adaptive functions of cognitive traits, it is current to seek for correlations between the occurrence of cognitive traits and ecological or neuroanatomical factors (see for instance [22]). In this search for correlations it is crucial to take into account the phylogeny when comparing several species: correlation tests assume independent points, but phylogenetically related species are not. Phylogeny can sometimes be a crucial factor to explain the distribution of characters, what can be called phylogenetic inertia.
Depending on the characters and species studied, the strength of phylogenetic inertia, or phylogenetic signal, can vary. MacLean et al. [10] detail the principle of one of the statistics measuring phylogenetic signal: lambda. Lambda ranges between 0 and 1 and indicates to what extent the covariation of two traits coincides with phylogeny. When lambda is near 0, the phylogenetic signal is low, thus the potential correlation between traits is independent of phylogeny (a confonding effect is that when there is no variation of the ability in the sample, lambda also equals 0, whereas it could reflect a shared feature at the sample scale). On the opposite, when lambda equals 1, the potential correlation fits perfectly with phylogeny (Figure 2). Lambda can then be used to rescale the internal branches of the phylogenetic tree to visualize more intuitively the phylogenetic signal. When it is high, the species points are not independent thus phylogenetic correction must be used for correlating traits. Two main methods exist: phylogenetic contrasts [23] or PGLM [24] (Figure 2).
MacLean et al. [10] also detail phylogenetic targeting (Figure 2): a method to optimally designate species (phylogenetically and statistically speaking) to investigate for a comparative study, given the results you already have on different species.
Figure 2. Different use of phylogeny for the study of phenotypic traits, including cognitive abilities.
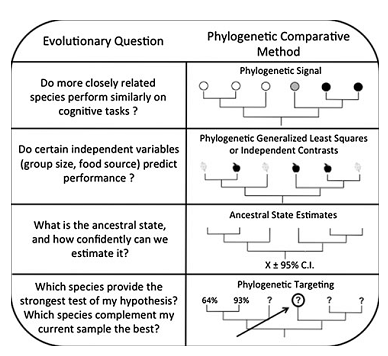
Phylogenetic signal is a measure of the importance of phylogeny in the distribution of a given trait. In the uppest example, phylogenetic signal should be quite high (lambda=1, see the text) but much lower in the second case. Phylogenetic signal depends on the scale of investigation. When searching for correlations between two traits, phylogeny must be taken into account because of a potential confounding effect of phylogeny. When knowing current states of modern species, ancestral state can be inferred (possibly with confidence intervals when states can be quantified) to retrace the evolutive history of the ability, by applying the principle of parsimony. This could help determine how much labile are cognitive traits in evolution. Finally, for comparative studies, phylogenetic targeting can help determine which species to investigate to statistically improve the dataset already collected. All those methods are presented in [10]) and this figure comes from their article. (Adapted from [10])
Establishing the evolutive history of a cognitive trait
When phylogenetic signal is high enough, it can be attempted to retrace the evolutive history of a cognitive trait: when and where did it appear? That is mapping characters on a phylogenetic tree established by other means (molecular or morphological) and infers ancestral state (Figure 2) This is the attempt of Fitch et al. [9] for investigating the evolutive origin of cognitive modules related to language ability, among which social learning is crucial.
How to state homology?
Considering that cognitive traits must have a heritable part, homologous cognitive characters should be determined. It could be difficult in practice for some characters to detect their origin, if the specialization is too high. It may also be hard to define clear distinct cognitive mechanisms, as they are interdependent with each other (but phylogenetic approach could help determining the strength of those relations, ie modularity). In a nutshell, homologies in cognitive mechanisms must exist but would be hard to define. Fitch et al. [9] takes convincing examples of some cognitive traits that could be homologous, even if the article emphasizes on the importance of convergence (or analogy). But they don't explain explicitly how to operationally define homologies.
In morphological studies, there are classically two successive steps to state homology of a character [7,25]. The primary homology is determined thanks to 3 criteria: position , specificity , and continuity (Figure 3). Two characters that occupy the same position in an organism, regardless of their function, might be homologous. This position criterion has been transposed for behavioral characters: two behavioral patterns occupying the same “place” in a more general behavior (Wenzel takes the example of tail-wagging within Tilapia courtship behavior [7]), even if not identical, might be homologous. Garcia [25] proposed to generalize this criterion for functional homologies, which could thus be used in theory for cognitive traits. The idea is to identify 2 functional traits (S1 and S2) performing a same function (F, possibly in different ways) which are each part of larger functional systems (T1 and T2 respectively), with T1 and T2 sharing also the same function F* and similar organization (Figure 3). The second criterion for primary homology, specificity , states that complex derived characters in two clades are likely to be homologous. It assumes that characters more generally evolve towards greater complexity. This criterion can be used for behavioral characters according to Wenzel [7], who takes the example of web building by spiders. Garcia [25] also considers this criterion could be used without modification for cognitive characters. Finally, the continuity argument is that if characters can be placed on a continuum of states, then they are probably homologous. Here again it has been proposed to use the same criterion for behavioral [7] or for cognitive [25] characters. When primary homologous characters are determined, secondary homology can be tested for a stronger assumption of homology. It consists in mapping the character on a phylogenetic tree, and to apply the principle of parsimony to check the common ancestry [7,25]. For cognitive abilities quantitatively assessed, McLean et al. [10] propose to use specific quantitative models to infer the common ancestor state (thus allowing confidence intervals to be calculated)
Figure 3. Three criteria for defining primary functional homology between functional characters S1, S2…S5.
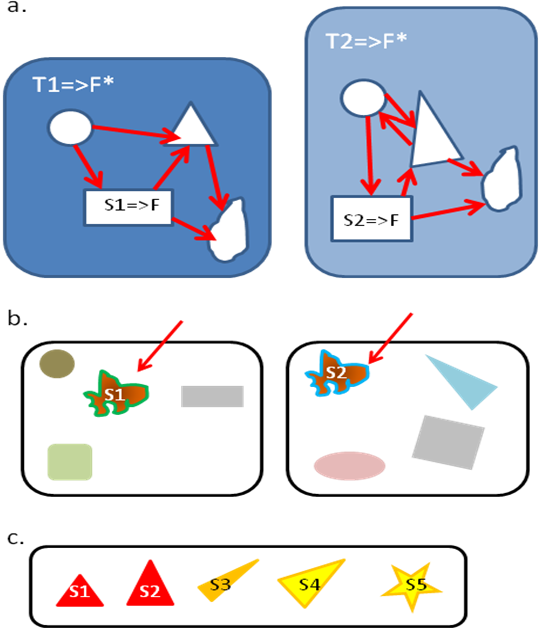
Homology is sameness by common descent. These criteria have been transposed from those designed for morphological and behavioral studies [25]. a. Position criterion (redrawn from [25]). S1 and S2 perform a same function F, and are part of larger functional system (respectively T1 and T2) which performs a same function F*. S1 and S2 share the same kind of relation with other parts of T1 and T2 (the same relative position), thus might be considered homologous. b. Specificity criterion. S1 and S2 are very specific and complex thus could be assumed to be homologous. c. Continuity criterion. S1 to S5 can be placed on a continuum thus are likely to be homologous. The more criteria the characters fulfill, the more robustly the primary homology can be inferred. By mapping characters on a tree, secondary homology can be tested (to further support homology of characters)
Plasticity of cognitive characters: how to infer absence of a capacity?
A major obstacle is that it is impossible to prove the absence of a capacity, a fortiori when the characters are very plastic like cognitive traits. To reduce the importance of this issue, McLean et al. [10] proposed to build a database synthesizing not only positive results for cognitive processes detected in particular species (in “standardized” experiments) but also the experiments that failed to provide evidence for a capacity. Absence could then be most reliably inferred when several different procedures failed to detect it in a species.
Are cognitive capacities too labile?
This phylogenetic approach could help bringing answers to an important debate in cognitive research. Two hypotheses are often adopted and confronted regarding cognitive abilities [3,26,27] the special adaptation hypothesis for which cognitive traits are primarily reflecting peculiar abilities of species for their niche, and too labile to be comparable between distant species ; and the general process hypothesis that says on the contrary that we can define broad-distributed cognitive abilities (eg associative learning, imitation…) that can be employed in different situations for different species, and thus comparable between distant species [26]. Papini [28] well explained the limits of this opposition, emphasizing on the fact that evolution is necessarily the result of both stasis and changes (see also [1] chapter 2). Each cognitive process would be the result of both inherited broad ability and more peculiar adjustments, but the relative importance of each is hardly known [27]. An illustration of this problem is the former mentioned two hypotheses for social learning: is it individual learning applied to social context (general-process) or special adaptation to sociality?
In Drosophila melanogaster , significative quantitative differences on several cognitive processes (Long and Short-term memory, Retroactive interference [29,30]) have been reported between 2 strains from a natural polymorphism (it seems that only one gene, for, is sufficient to cause those differences [29,30]). Thus changes could be easy to occur in some cases at least, but sometimes cognitive abilities appear quite stable (see Box 1 and the vocal imitation in Birds). We argue that phylogenetic mapping could help determining the lability rate(s), that is to say testing this hypothesis for different taxa and processes. To do so, beginning with narrower phylogenetic analysis could be less controversial. Assessing the phylogenetic signal in the data can help determine whether it is worth the effort [10].
Furthermore it is crucial to remember two things, both explained by Ereshefsky [31]: homologous characters are not necessarily identical, but are similar by common descent; and secondly, homology is depending on the scale you are looking at. If phenotypic characters can be seen as homologies within certain clades, their determinisms at organ, cellular and/or genetic levels might not be (e.g. Eukaryotes have genders determined in various ways). The reverse is also true, so even if neuronal mechanisms are shared between learning processes, this does not necessarily mean homologous learning abilities. For a more documented analysis of conservation of gene function in behavior see the namely article by Reaume and Sokolowsky [32], which also tackles the case of genes involved in learning and memory among animals (including the previously mentioned for and its homologs). A consequence of this is that if reconstructing evolutive history of cognitive traits could contribute to determine those worth to be investigated for their mechanisms (as De Waal et Ferrari call to [33]), it does not necessarily mean that mechanisms will be entirely shared by all organisms [32].
Phylogenetic studies are just beginning in the field of cognitive sciences but could bring complementary information to the classical mechanistic or adaptative focus. Methods have been proposed for defining homologies, perform analyses (to assess ancestral state or phylogenetic signal). Given the influence social learning could have on evolutionary processes, and its importance in human societies, we hope this approach will develop.
Acknowledgments
I would like to thank Frederic Mery for his useful corrections and comments during the elaboration of this report, and Didier Casane for ideas and clarifications on the potential of phylogeny.
References and recommended reading
Papers of particular interest have been highlighted as:
● of special interest
●● of outstanding interest
2. Shettleworth SJ: Animal cognition and animal behaviour . Animal Behaviour 2001, 61 :277–286. [ Full Paper ]
3. Dukas R, Dukas R, Ratcliffe JM: Learning mechanisms, ecology, and evolution . Cognitive ecology II 2009, [no volume].
4. Fragaszy DM, Perry S: Towards a biology of traditions . The biology of traditions: models and evidence 2003, [no volume].
5. Hoppitt W, Laland KN: Chapter 3 Social Processes Influencing Learning in Animals: A Review of the Evidence [Internet] . In Advances in the Study of Behavior . Edited by H. Jane Brockmann TJR. Academic Press; 2008:105–165.
6. Tinbergen N: On aims and methods of Ethology . Zeitschrift für Tierpsychologie 1963, 20 :410–433. [ Full Paper ]
7. Wenzel JW:
Behavioral homology and phylogeny
.
Annual review of ecology and systematics
[date unknown],
23
:361–381.
●● This article was among those which brought the phylogeny back in studies of behavior in the 90's. It proposed explicitly for the first time to transpose the criteria for primary homology used in morphological phylogeny, for behavioral traits. The explanation is clear and it provides illustrative examples.
8. Price JJ, Clapp MK, Omland KE: Where have all the trees gone? The declining use of phylogenies in animal behaviour journals . Animal Behaviour 2011, 81 :667.
9. Fitch WT, Huber L, Bugnyar T:
Social cognition and the evolution of language: constructing cognitive phylogenies
.
Neuron
2010,
65
:795–814. [
Full Paper
][
PubMed
]
●● Phylogeny of cognitive abilities are very rare, but this article try to adopt this point of view for analyzing the evolutive origin of cognitive modules that can be thought to be involved with human language ability. There is no specific phylogenetic methodology used but this work provides convincing examples of cognitive traits that could be homologies.
10. MacLean EL, Matthews LJ, Hare BA, Nunn CL, Anderson RC, Aureli F, Brannon EM, Call J, Drea CM, Emery NJ, et al.:
How does cognition evolve? Phylogenetic comparative psychology
.
Anim Cogn
2012,
15
:223–238. [
Full Paper
][
PubMed
]
●● Probably the central article for the forthcoming cognitive phylogeny studies; it reviews methods that can be used for phylogenetic studies. Interestingly, the numerous authors propose to create a devoted database to compile all works, that will be done with standardized protocols. They cover a large range of potential use of phylogeny.
11. KENDAL RL, COOLEN I: Adaptive Trade-offs in the Use of Social and Personal Information . Cognitive ecology II 2009, [no volume].
12. Laland KN, Kendal JR: What the models say about social learning . The biology of traditions: Models and evidence 2003, [no volume].
13. Cavalli-Sforza LL, Feldman MW: Cultural Transmission and Evolution: A Quantitative Approach.(MPB-16) [Internet] . Princeton University Press; 1981.
14. Danchin É, Charmantier A, Champagne FA, Mesoudi A, Pujol B, Blanchet S: Beyond DNA: integrating inclusive inheritance into an extended theory of evolution . Nat. Rev. Genet. 2011, 12 :475–486. [ Full Paper ][ PubMed ]
15. Laland KN, Odling-Smee J, Myles S: How culture shaped the human genome: bringing genetics and the human sciences together . Nat. Rev. Genet. 2010, 11 :137–148. [ Full Paper ][ PubMed ]
16. Rendell L, Mesnick SL, Dalebout ML, Burtenshaw J, Whitehead H: Can genetic differences explain vocal dialect variation in sperm whales, Physeter macrocephalus ? Behav. Genet. 2012, 42 :332–343. [ Full Paper ][ PubMed ]
17. Mery F, Varela SAM, Danchin É, Blanchet S, Parejo D, Coolen I, Wagner RH:
Public Versus Personal Information for Mate Copying in an Invertebrate
.
Current Biology
2009,
19
:730–734. [
Full Paper
]
● Together with the following reference, those two articles elegantly suggest that tradition and cultural transmission could occur in unexpected taxa, and furthermore place Drosophila as a major model for this field of research.
18. Battesti M, Moreno C, Joly D, Mery F: Spread of social information and dynamics of social transmission within Drosophila groups . Curr. Biol. 2012, 22 :309–313. [ Full Paper ][ PubMed ]
19. Fiorito G, Scotto P: Observational Learning in Octopus vulgaris . Science 1992, 256 :545–547. [ Full Paper ][ PubMed ]
20. Reader SM, Lefebvre L: Social learning and sociality . Behavioral and brain sciences 2001, 24 :353–355.
21. Wilkinson A, Kuenstner K, Mueller
J, Huber L:
Social learning in a non-social reptile
(
Geochelone carbonaria
)
.
Biol.
Lett.
2010,
6
:614–616. [
Full
Paper
][
PubMed
]
● An interesting article which studies social learning in a previously
unexplored group (non-avian Reptiles) and moreover in a non-social species,
testing an important hypothesis (social learning for social animals).
Unfortunately authors do not discuss the potential role of turtles
phylogeny.
22. Reader SM: Relative brain size and the distribution of innovation and social learning across the nonhuman primates . 2003, [no volume].
24. Pagel M: Inferring the historical patterns of biological evolution . Nature 1999, 401 :877–884. [ Full Paper ][ PubMed ]
25. García CL: Functional Homology and Functional Variation in Evolutionary Cognitive Science . Biological Theory 2010, 5 :124–135. [ Full Paper ]
26. Macphail EM, Bolhuis JJ: The evolution of intelligence: adaptive specializations versus general process . Biol Rev Camb Philos Soc 2001, 76 :341–364. [ PubMed ]
27. Bolhuis JJ, Wynne CDL: Can evolution explain how minds work? Nature 2009, 458 :832–833. [ Full Paper ][ PubMed ]
28. Papini MR: Pattern and process in the evolution of learning . Psychol Rev 2002, 109 :186–201. [ PubMed ]
29. Mery F, Belay AT, So AK-C, Sokolowski MB, Kawecki TJ: Natural polymorphism affecting learning and memory in Drosophila . Proc. Natl. Acad. Sci. U.S.A. 2007, 104 :13051–13055. [ Full Paper ][ PubMed ]
30. Reaume CJ, Sokolowski MB, Mery F:
A natural genetic polymorphism affects retroactive
interference in
Drosophila melanogaster
.
Proc. Biol. Sci.
2011,
278
:91–98. [
Full
Paper
][
PubMed
]
● The two preceding references illustrate that quantitatively, significant
differences in major cognitive ability can occur between individuals of the same
population, due to a natural polymorphism. It establishes strong link with
genetics and also illustrates that cognitive abilities can sometimes be very
labile.
31. Ereshefsky M: Psychological Categories as Homologies: Lessons From Ethology . Biology and Philosophy 2007, 22 :659–674.
32. Reaume CJ, Sokolowski MB: Conservation of gene function in behaviour . Philos. Trans. R. Soc. Lond., B, Biol. Sci. 2011, 366 :2100–2110. [ Full Paper ][ PubMed ]
33. de Waal FBM, Ferrari PF: Towards a bottom-up perspective on animal and human cognition . Trends Cogn. Sci. (Regul. Ed.) 2010, 14 :201–207. [ Full Paper ][ PubMed ]
34. Suh A, Paus M, Kiefmann M, Churakov G, Franke FA, Brosius J, Kriegs JO, Schmitz J:
Mesozoic retroposons reveal parrots as the closest living relatives of passerine birds
.
Nat Commun
2011,
2
:443. [
Full Paper
][
PubMed
]
● This study strongly supports previous results for Parrots as sister group of Passerines with an independent method, and the authors discuss the implications for the evolutive history of vocal learning.
35. Clayton DF, Balakrishnan CN, London SE: Integrating genomes, brain and behavior in the study of songbirds . Curr. Biol. 2009, 19 :R865–873. [ Full Paper ][ PubMed ]
36. Saranathan V, Hamilton D, Powell GVN, Kroodsma DE, Prum RO: Genetic evidence supports song learning in the three-wattled bellbird Procnias tricarunculata ( Cotingidae ) . Mol. Ecol. 2007, 16 :3689–3702. [doi: 10.1111/j.1365-294X.2007.03415.x] [ PubMed ]
37. Hackett SJ, Kimball RT, Reddy S, Bowie RCK, Braun EL, Braun MJ, Chojnowski JL, Cox WA, Han K-L, Harshman J, et al.:
A phylogenomic study of birds reveals their evolutionary history
.
Science
2008,
320
:1763–1768. [
Full Paper
][
PubMed
]
A major contribution to the understanding of the relations between Birds Orders. This study was the first to propose Parrots as sister-group of Passerines, implying that vocal imitation could have appeared in the common lineage of those two major orders, and not twice independently as previously thought.
38. Pacheco MA, Battistuzzi FU, Lentino M, Aguilar RF, Kumar S, Escalante AA: Evolution of modern birds revealed by mitogenomics: timing the radiation and origin of major orders . Mol. Biol. Evol. 2011, 28 :1927–1942. [ Full Paper ][ PubMed ]
39. Haesler S, Wada K, Nshdejan A, Morrisey EE, Lints T, Jarvis ED, Scharff C: FoxP2 expression in avian vocal learners and non-learners . J. Neurosci. 2004, 24 :3164–3175. [ Full Paper ][ PubMed ]



